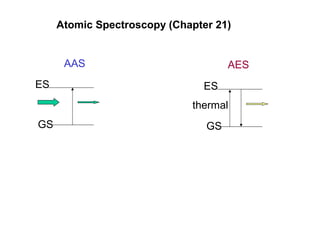
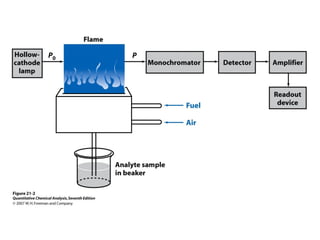
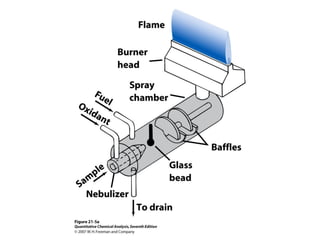
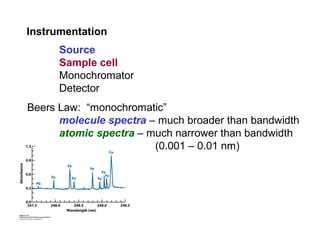
Atomic spectroscopy is used for qualitative and quantitative elemental analysis. It involves converting a sample into atoms, exciting the atoms, and measuring their absorption or emission of light. There are three main types of atomic spectroscopy: absorption, emission, and fluorescence spectroscopy. Samples are atomized using different heat sources like flames, furnaces, or plasma which convert the sample into gas phase atoms. The temperature of the heat source impacts the population of atoms in ground, excited, and ionized states. Instrumentation includes an atomization source, sample cell, monochromator, and detector. Detection limits range from parts-per-million to parts-per-trillion depending on the element and method used.