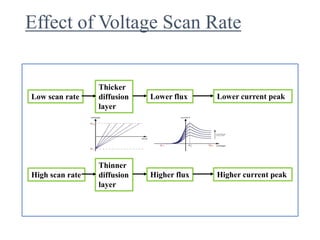
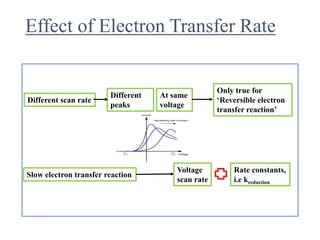
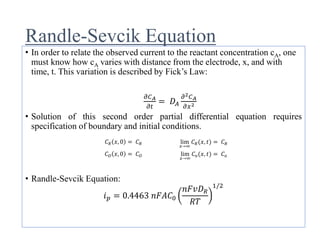
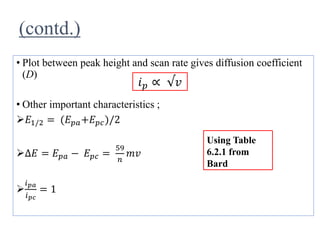
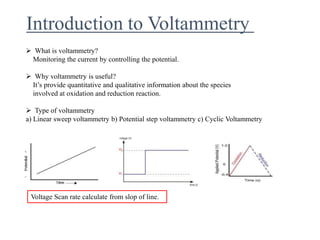
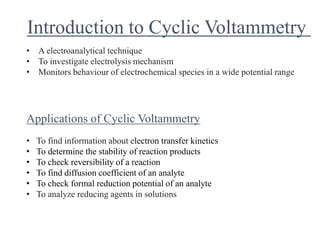
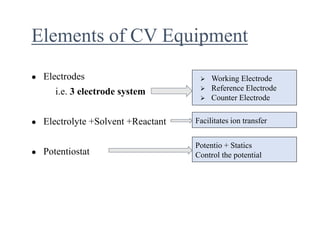
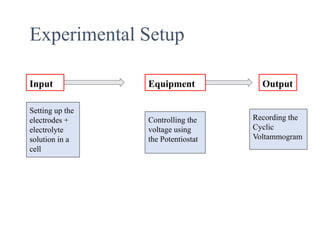
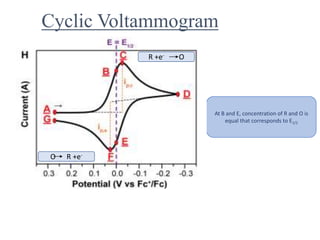
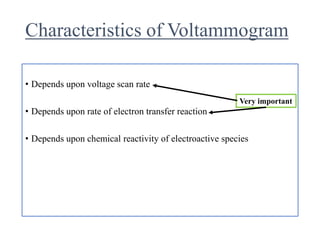
This document provides an overview of cyclic voltammetry, an electroanalytical technique used to study electrochemical reactions by monitoring current as a function of applied potential. It discusses various applications, key components of the setup such as the three-electrode system, and the significance of the cyclic voltammogram in analyzing electron transfer kinetics and reaction stability. Additionally, it covers the effects of scan rate and diffusion on the current response, and introduces essential equations like Fick's law and the Randle-Sevcik equation related to the study of voltammetry.

![Effect of Diffusion Layer
• Nernst equation ; E = E0 +
𝑹.𝑻
𝒏.𝑭
. 𝒍𝒏
[𝑹𝒆𝒂𝒄𝒕𝒂𝒏𝒕]
[𝑷𝒓𝒐𝒅𝒖𝒄𝒕]
Scanning of voltage Rise in current
Conversion
of
reactants
Increase
in
diffusion
layer
Sufficient growth in
diffusion layer
Rate of reaction ≠ Flux
A peak in current
and drop](https://image.slidesharecdn.com/pptppt-190924183829/85/Cyclic-Voltametery-8-320.jpg)