Embed presentation
Downloaded 59 times

![To calculate how much is in each phase,
K
Volume of organic phase
Volume of aqueous phase
Initial concentration in aqueous phase
n
orgaq
aq
KVV
V
q )(
+
=
[ ] ( ) [ ]A
V
V V K
Aaq n
aq
aq org
n
aq=
+
0
n = # times](https://image.slidesharecdn.com/solventextraction-140711154150-phpapp02/85/Solvent-extraction-5-320.jpg)
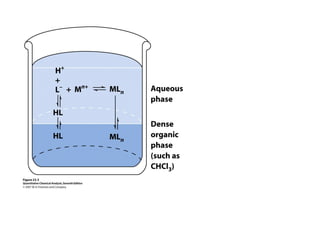
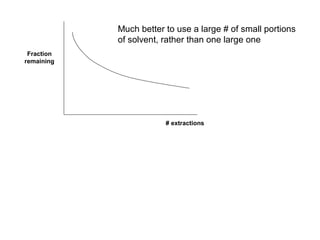
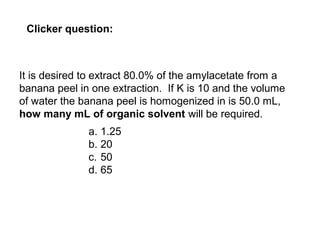
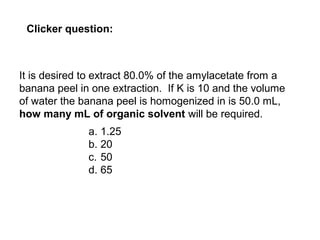
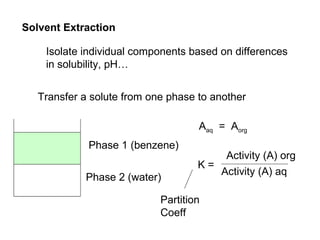
1) Solvent extraction is a technique used to separate components in a mixture based on differences in solubility between two immiscible liquid phases. 2) It involves transferring a solute from one liquid phase to another, such as transferring a compound from an aqueous phase to an organic phase like benzene. 3) The amount of solute extracted into each phase can be calculated using the partition coefficient K, which is a ratio of concentrations in the two phases at equilibrium.




![To calculate how much is in each phase,
K
Volume of organic phase
Volume of aqueous phase
Initial concentration in aqueous phase
n
orgaq
aq
KVV
V
q )(
+
=
[ ] ( ) [ ]A
V
V V K
Aaq n
aq
aq org
n
aq=
+
0
n = # times](https://image.slidesharecdn.com/solventextraction-140711154150-phpapp02/85/Solvent-extraction-5-320.jpg)




