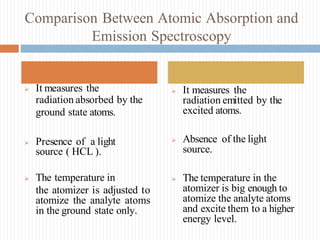
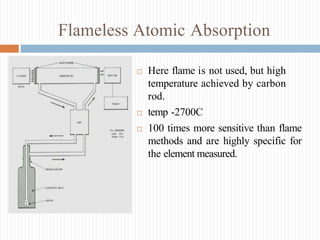
This document discusses atomic absorption spectroscopy and flame emission spectroscopy. It begins by explaining the basic principles of atomic absorption spectroscopy, where ground state atoms absorb radiation from a light source in a flame. It then describes the typical instrumentation used, including the light source, burner, monochromator, detector, and readout device. Applications include clinical analysis and environmental monitoring. Flame emission spectroscopy is also covered, noting that excited atoms emit radiation characteristic of the element. Both techniques can be used to measure trace metal concentrations, though they differ in whether they detect absorbed or emitted radiation. Advantages and disadvantages of each method are provided.