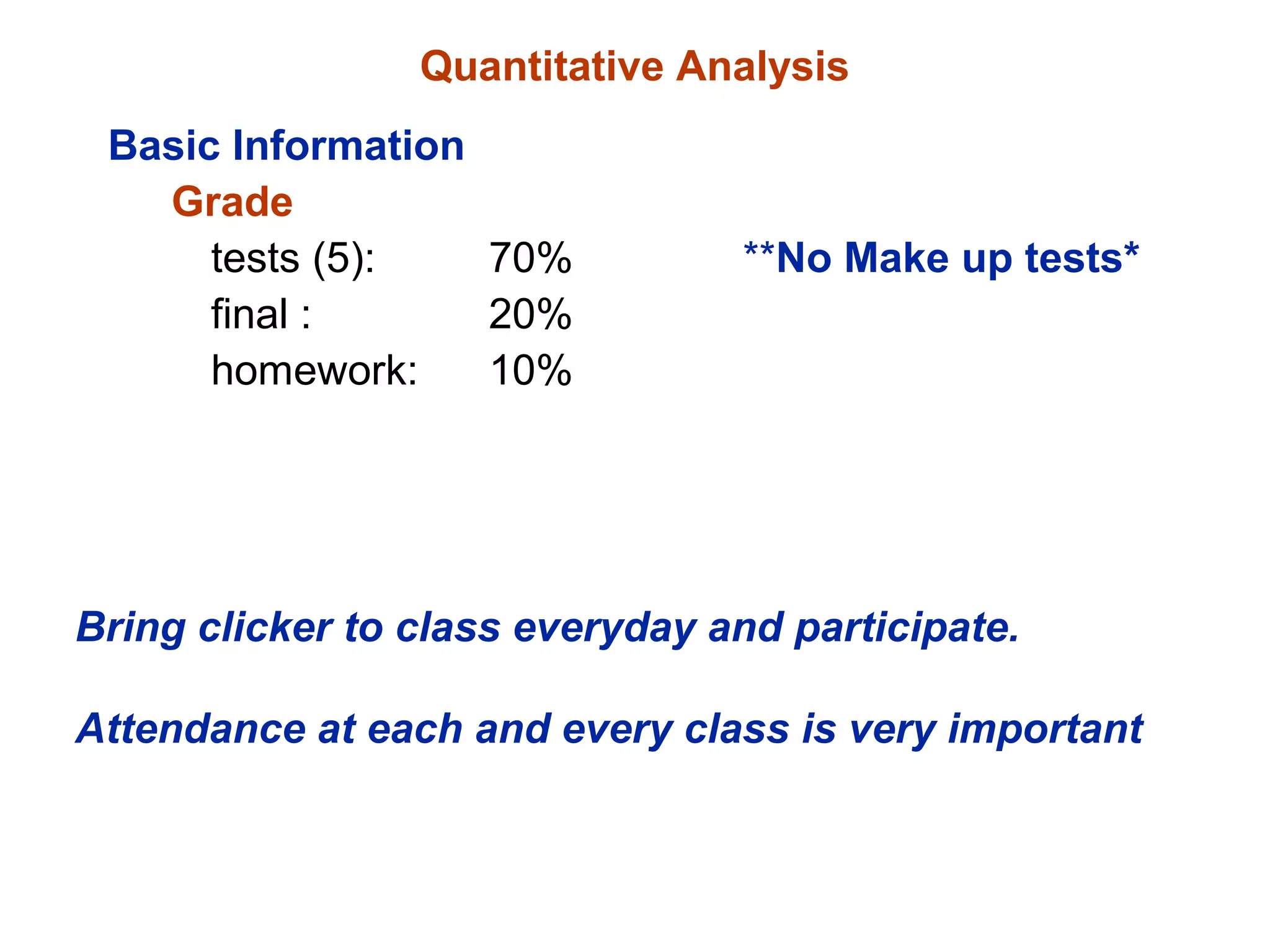
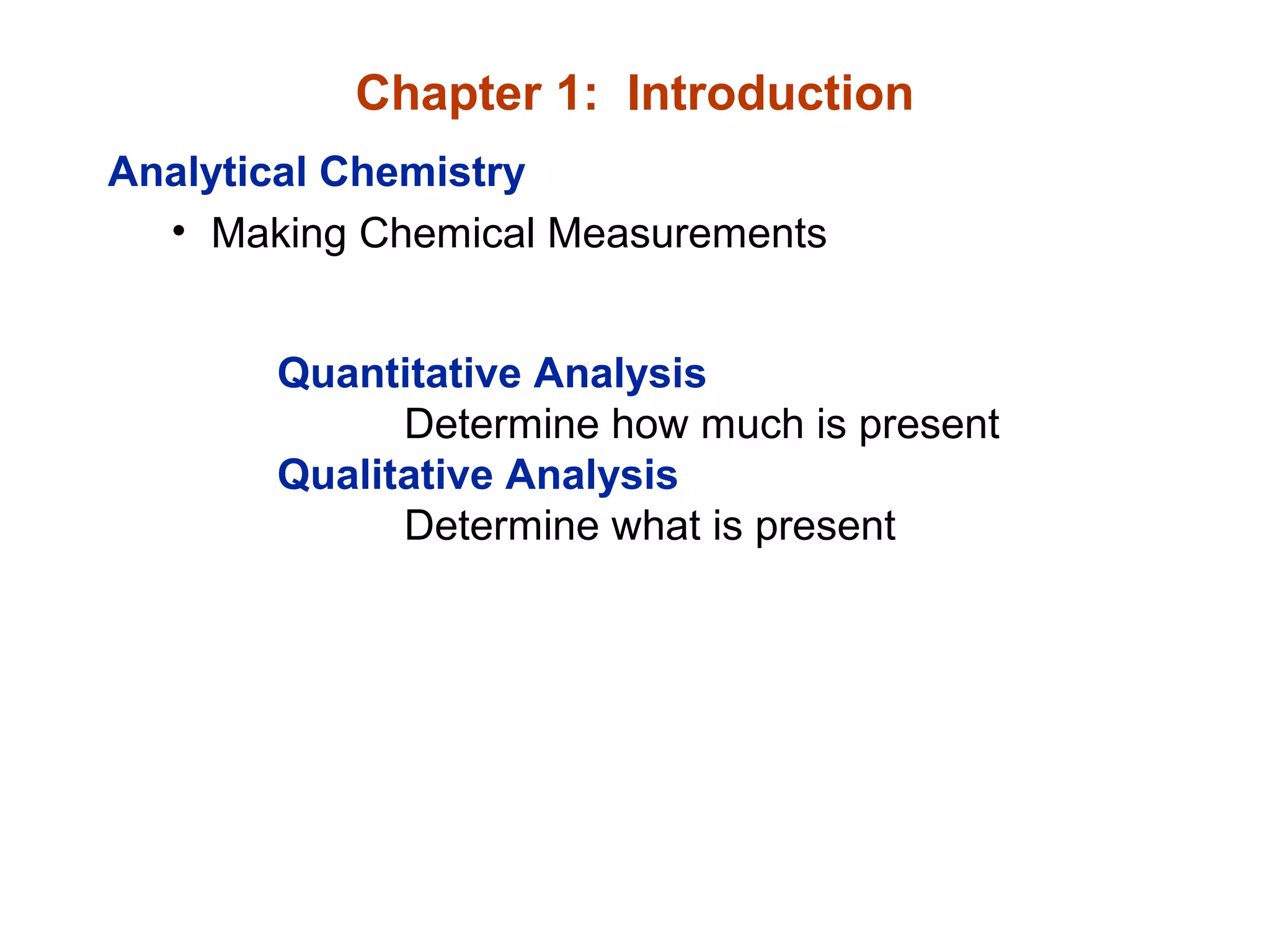
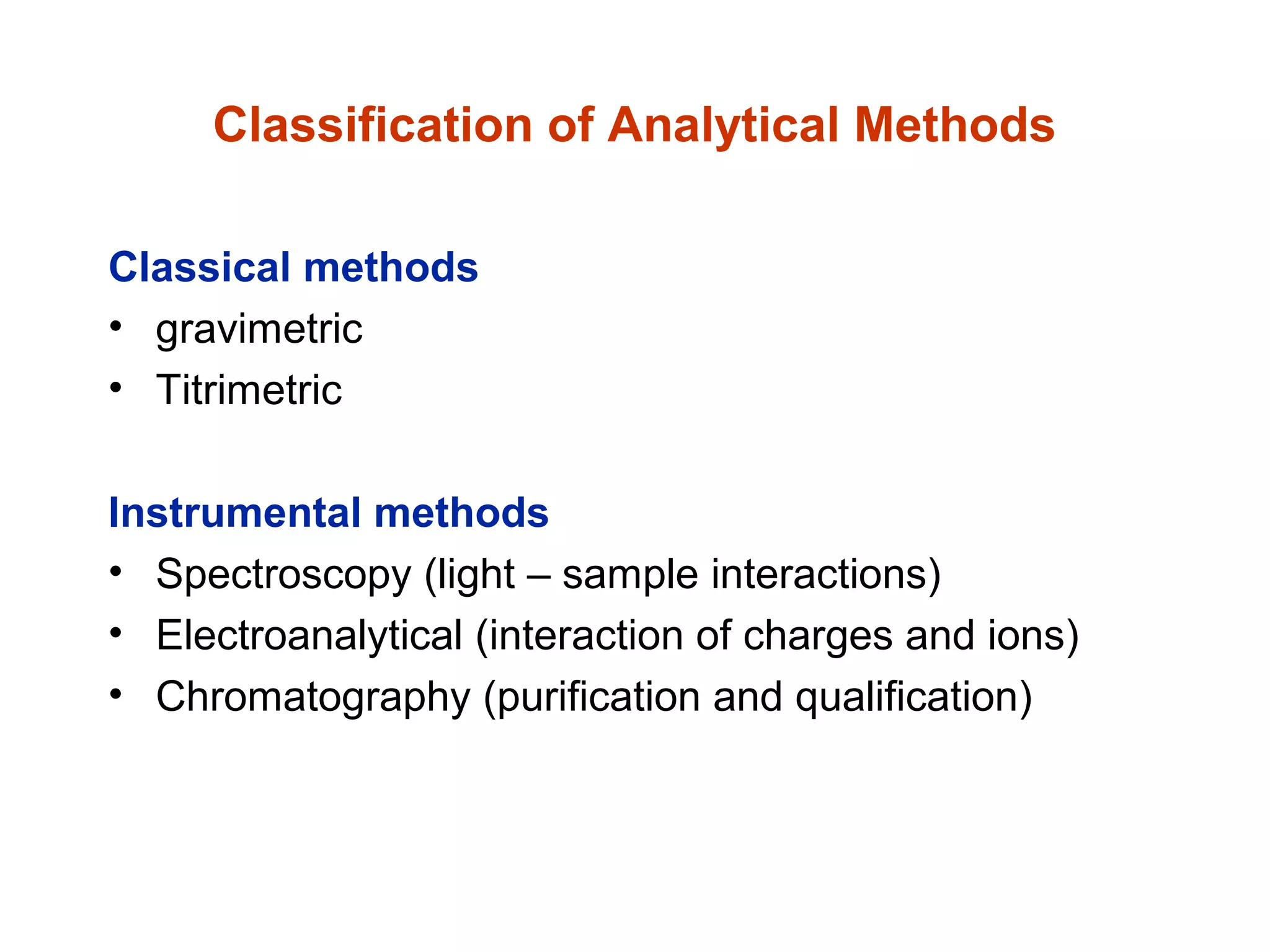
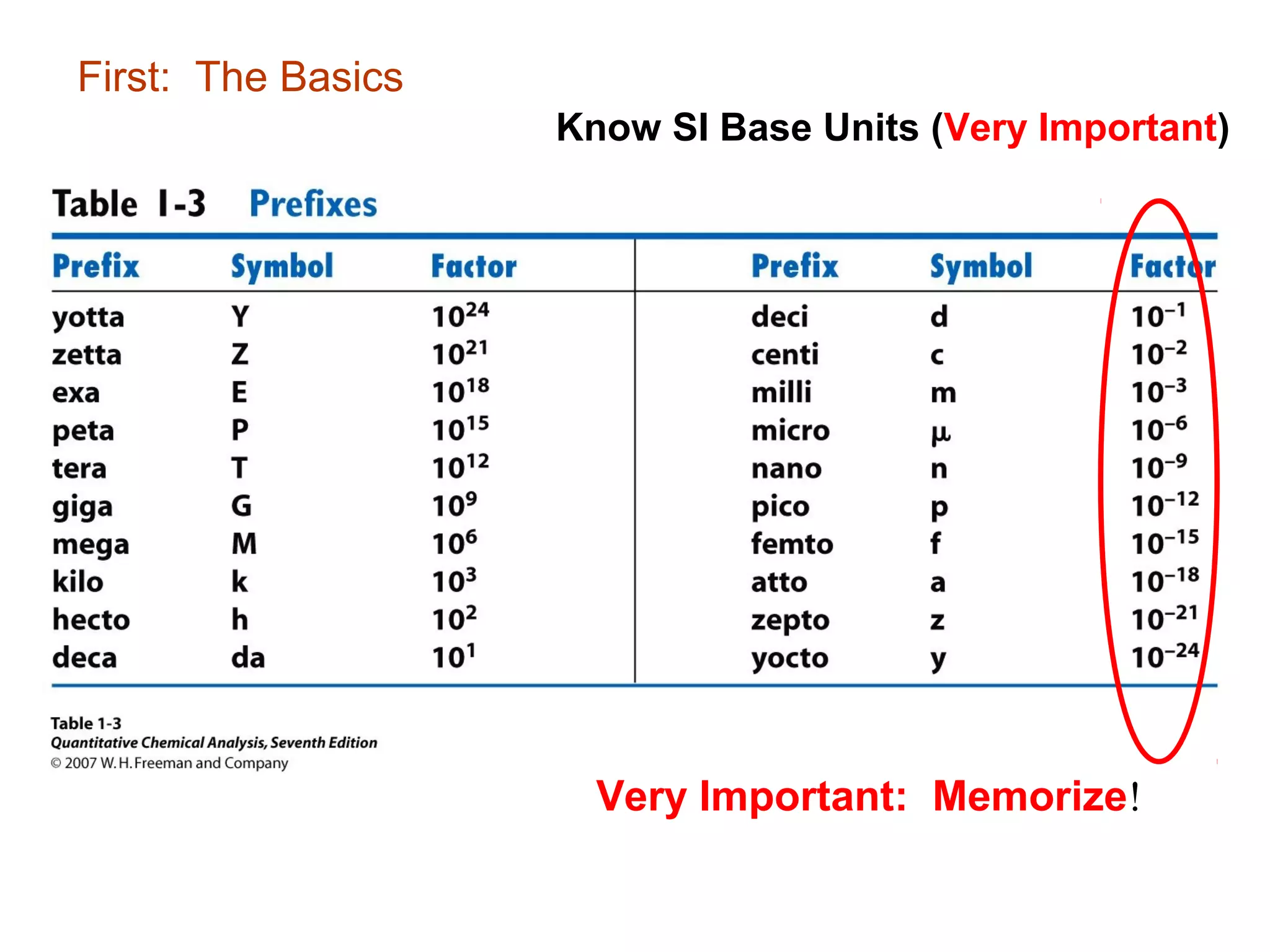
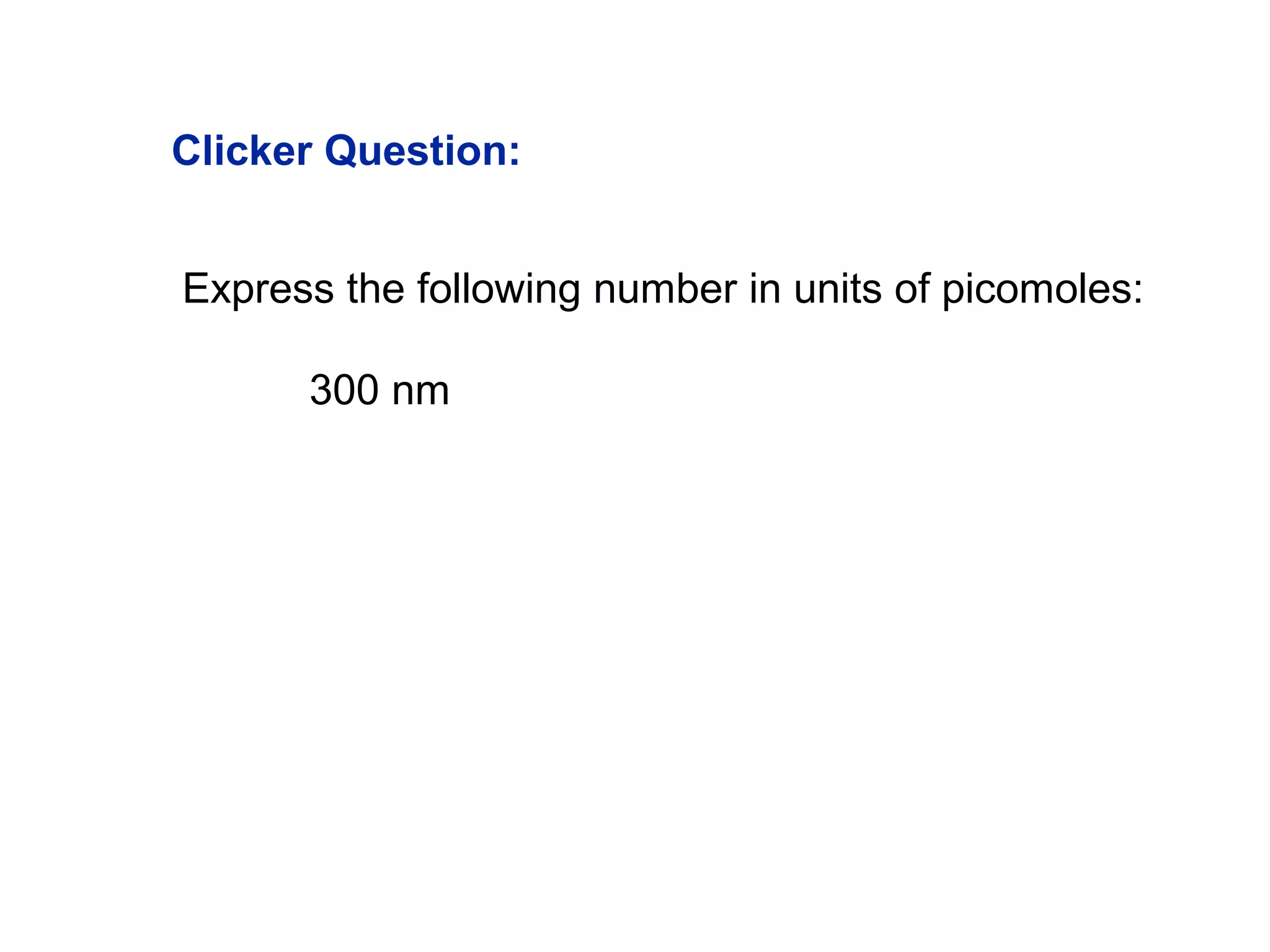
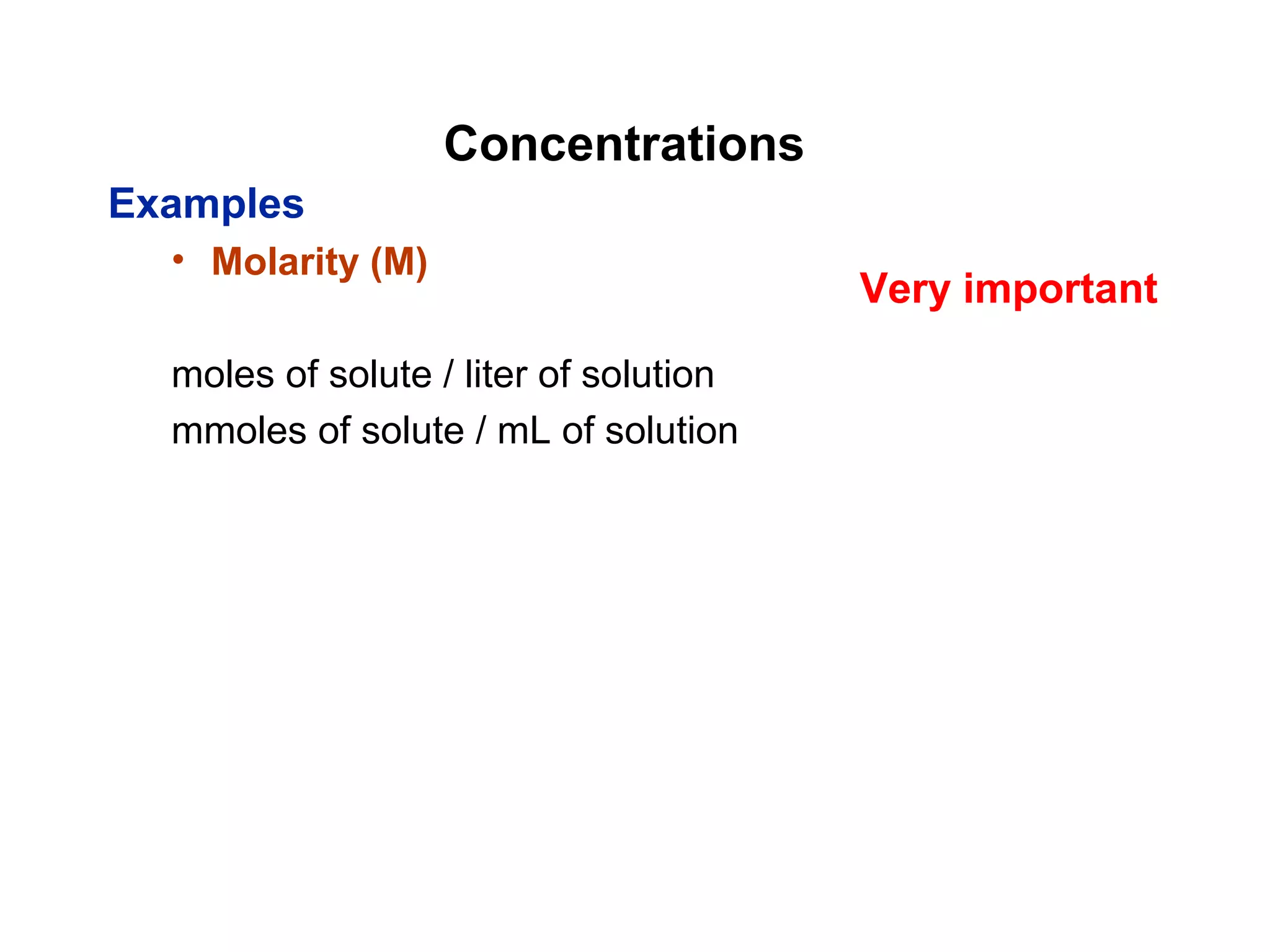
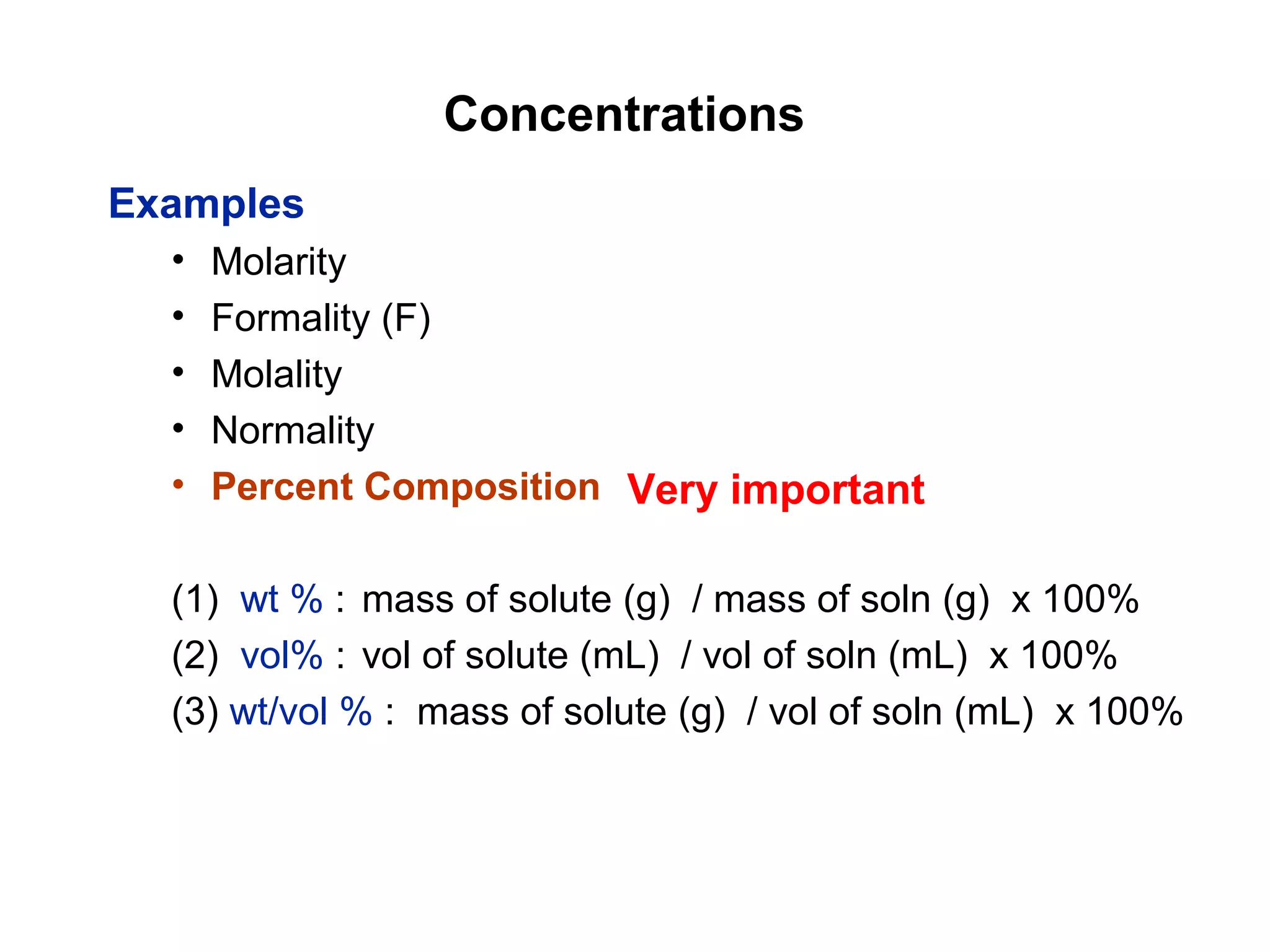
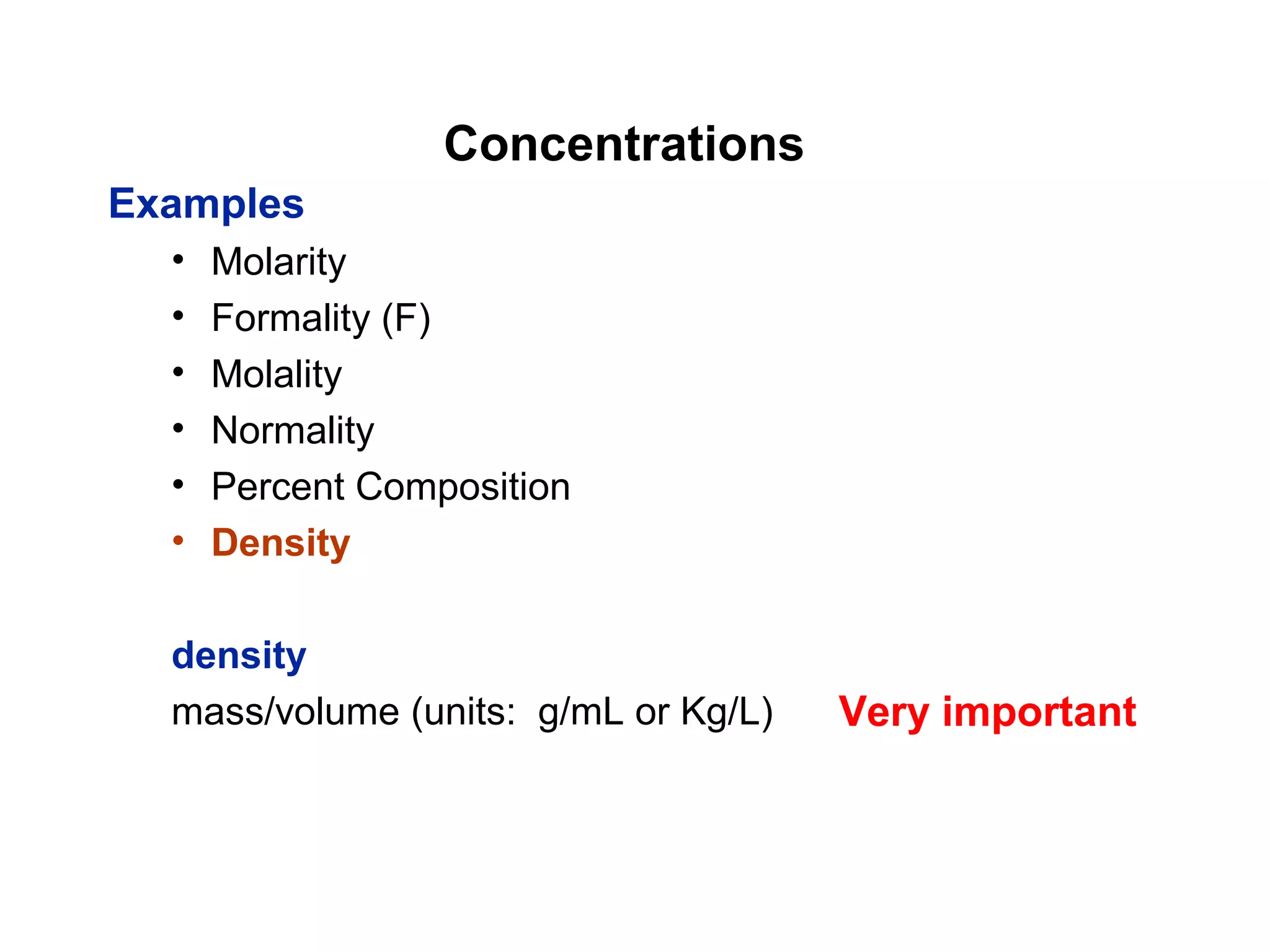
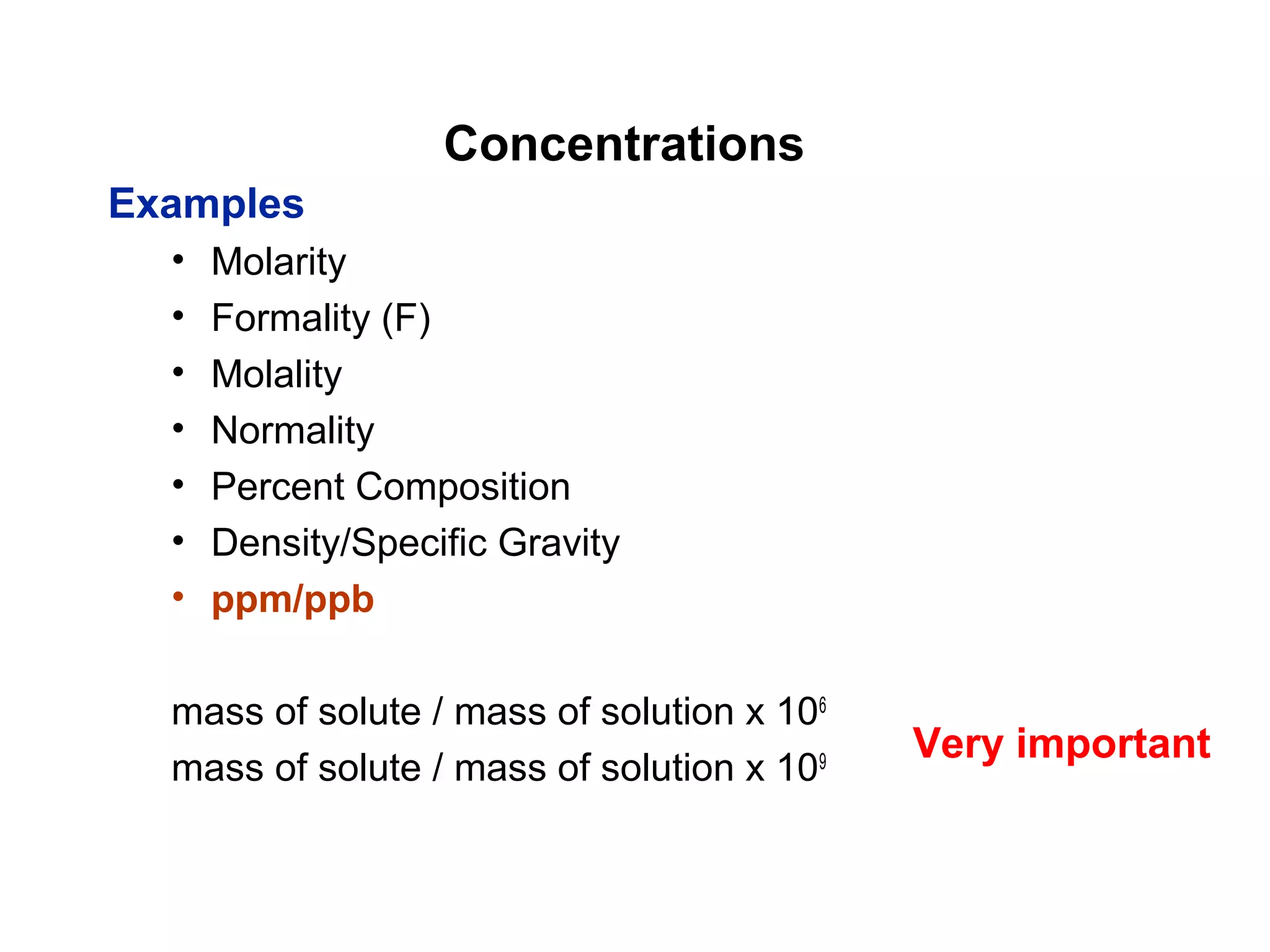
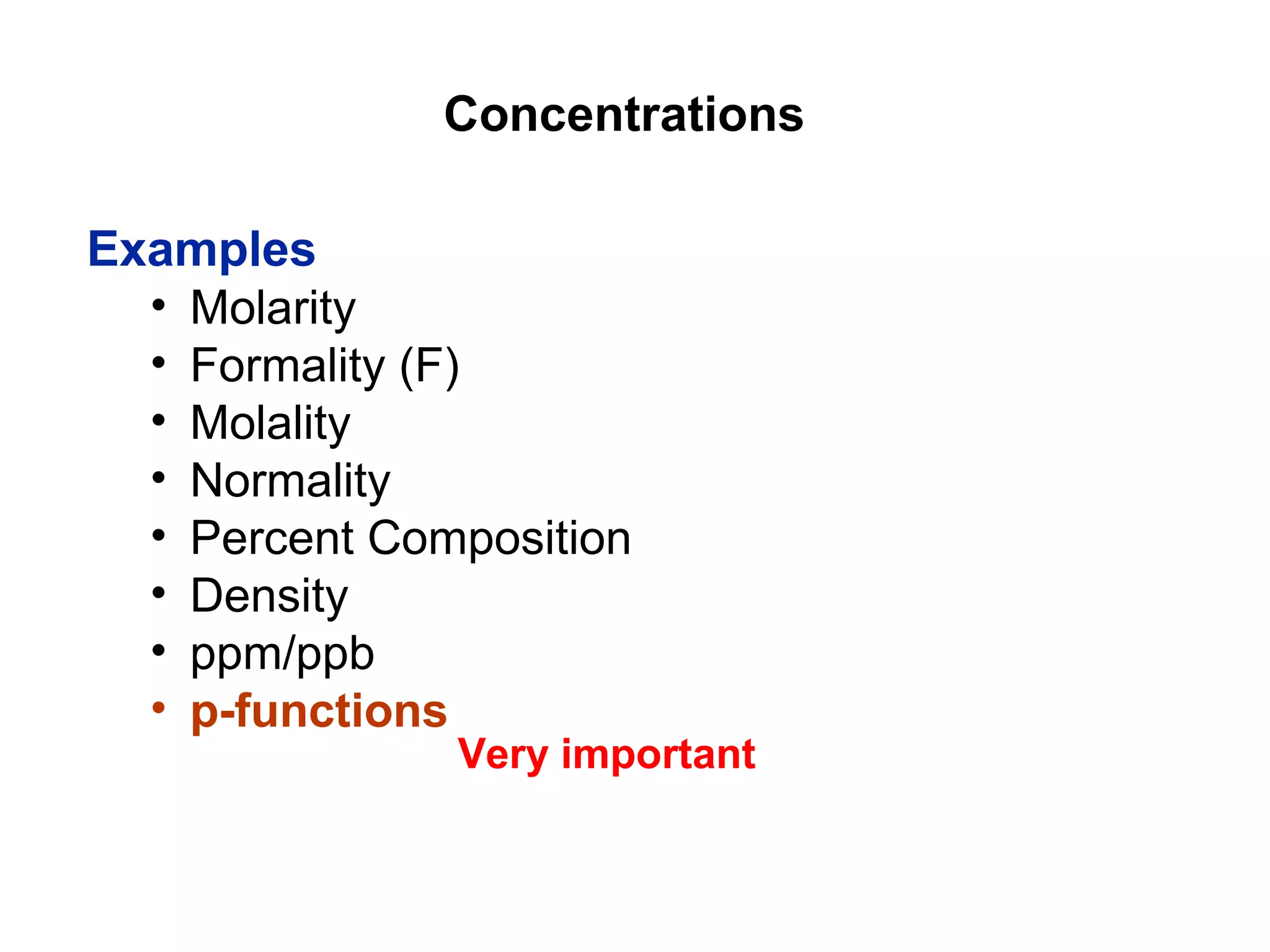
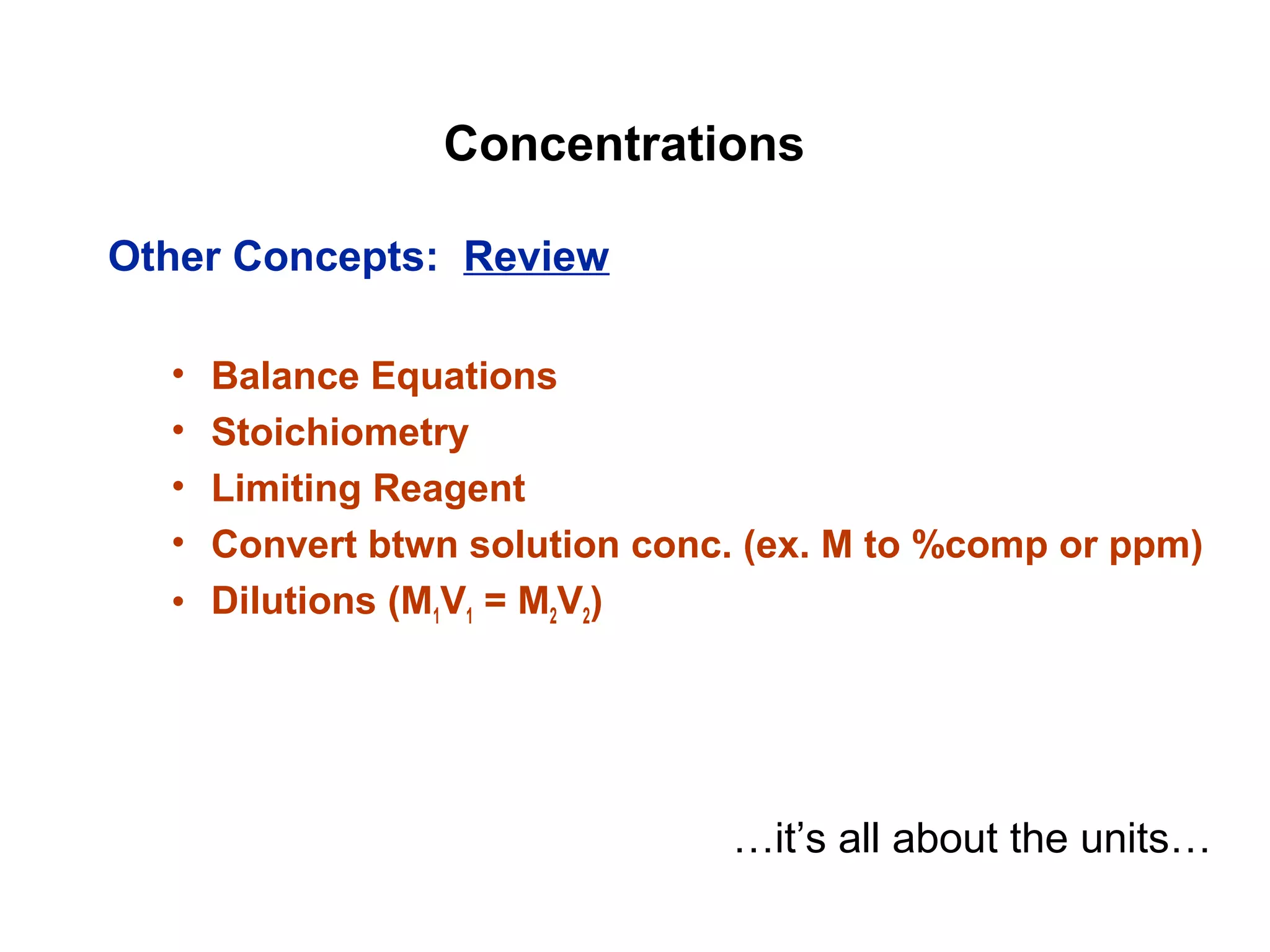
This document provides information about the Quantitative Analysis (CHEM 309) course including the instructor, textbook, grade breakdown, class objectives, and chapter overview. The grade is based on tests (70%), final (20%), and homework (10%). Students are expected to attend every class, participate daily with a clicker, and complete homework each night. The course covers topics like acid-base chemistry, analytical techniques, chemical measurements, and error analysis. Concentration units like molarity, formality, molality, and ppm/ppb are also discussed.