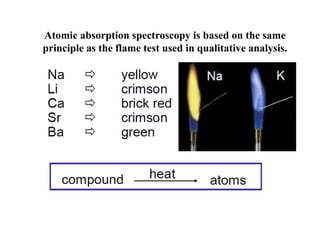
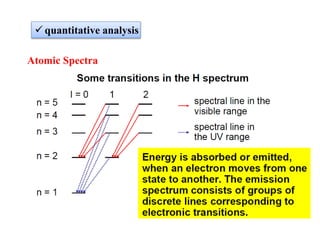
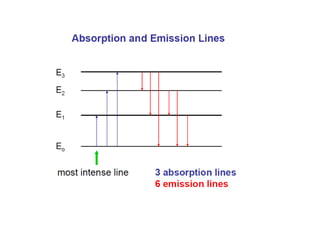
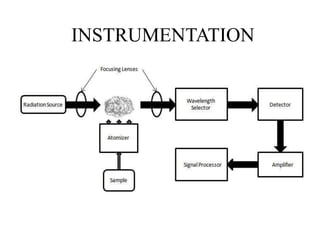
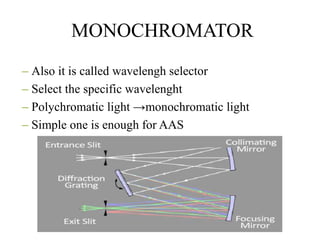
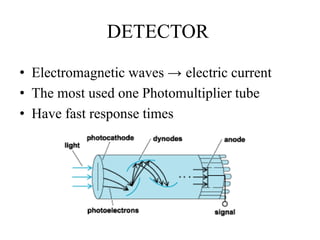
Atomic absorption spectroscopy (AAS) is a method for quantitatively determining chemical elements using light absorption by free atoms. Introduced by Alan Walsh in 1955, AAS has applications in fields such as mining, medicine, and agriculture, and is known for its high sensitivity and accuracy in analyzing various sample types. The technique involves the use of specific light sources, atomization methods, and calibration techniques to measure element concentrations effectively.
















![CONCLUSION
• There are many advantages
High sensitivity
[10-10 g (flame), 10-14 g (non-flame)]
Good accuracy
(Relative error 0.1 ~ 0.5 % )
High selectivity](https://image.slidesharecdn.com/aas-190208133004/85/Atomic-Absorption-Spectroscopy-33-320.jpg)
