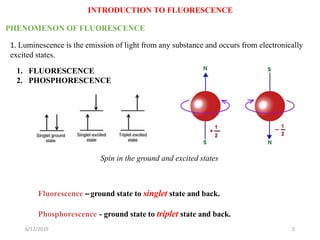
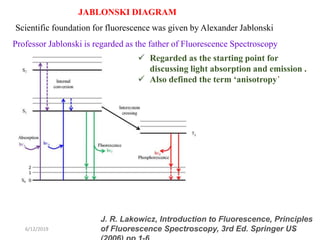
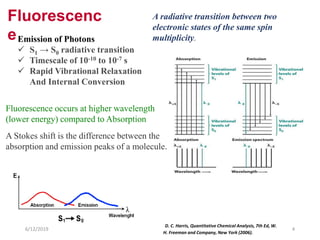
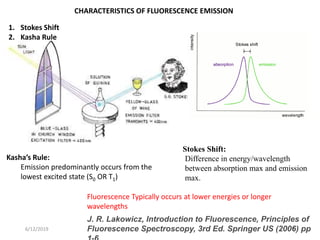
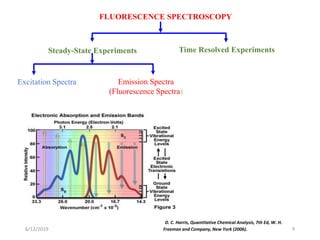
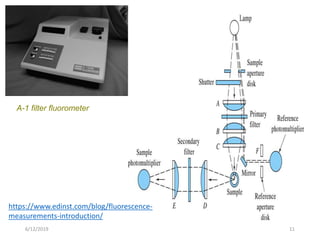
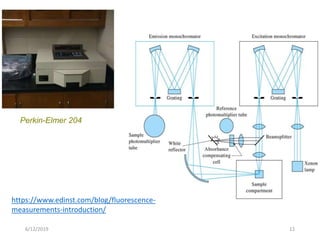
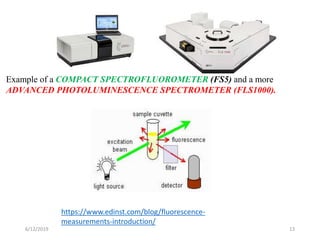
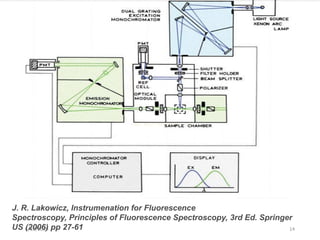
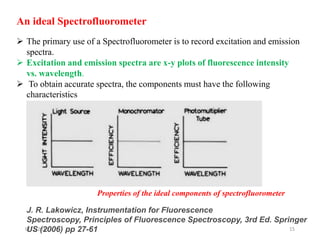
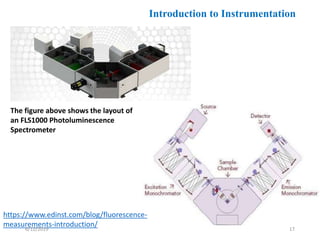
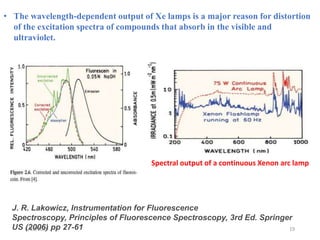
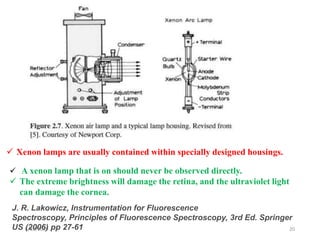
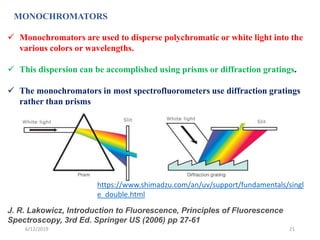
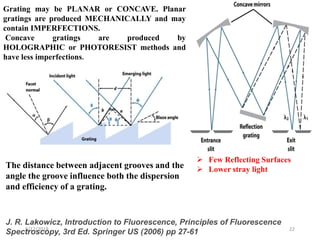
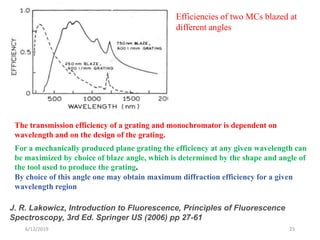
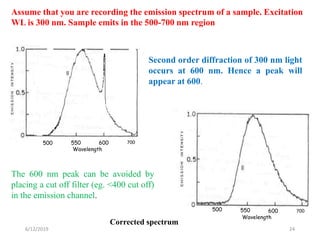
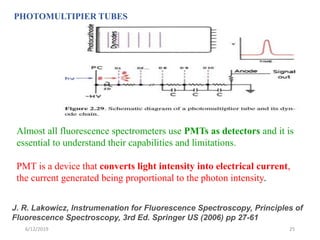
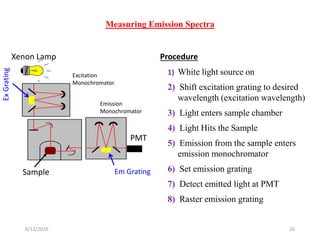
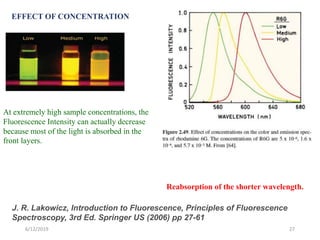
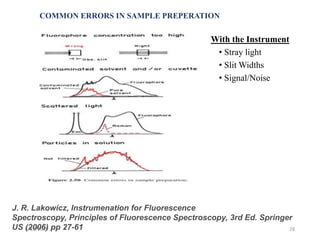
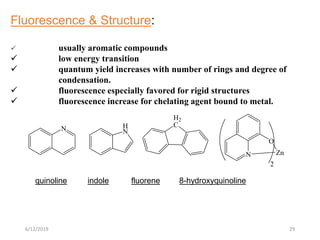
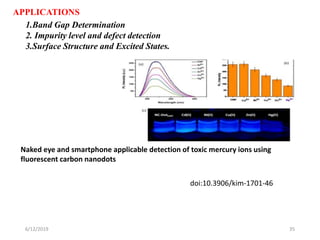
This document provides an overview of fluorescence spectroscopy. It begins with a brief introduction to fluorescence as a type of luminescence involving emission of light from electronically excited states. It then discusses the Jablonski diagram, which provides the scientific foundation for fluorescence. Several key characteristics of fluorescence emission are described, including Stokes shift and Kasha's rule. The document outlines some common applications of fluorescence spectroscopy and describes the basic components and operation of fluorescence spectrometers, including light sources, monochromators, and photomultiplier tubes. It concludes by noting that fluorescence intensity can decrease at extremely high sample concentrations due to factors like self-quenching.