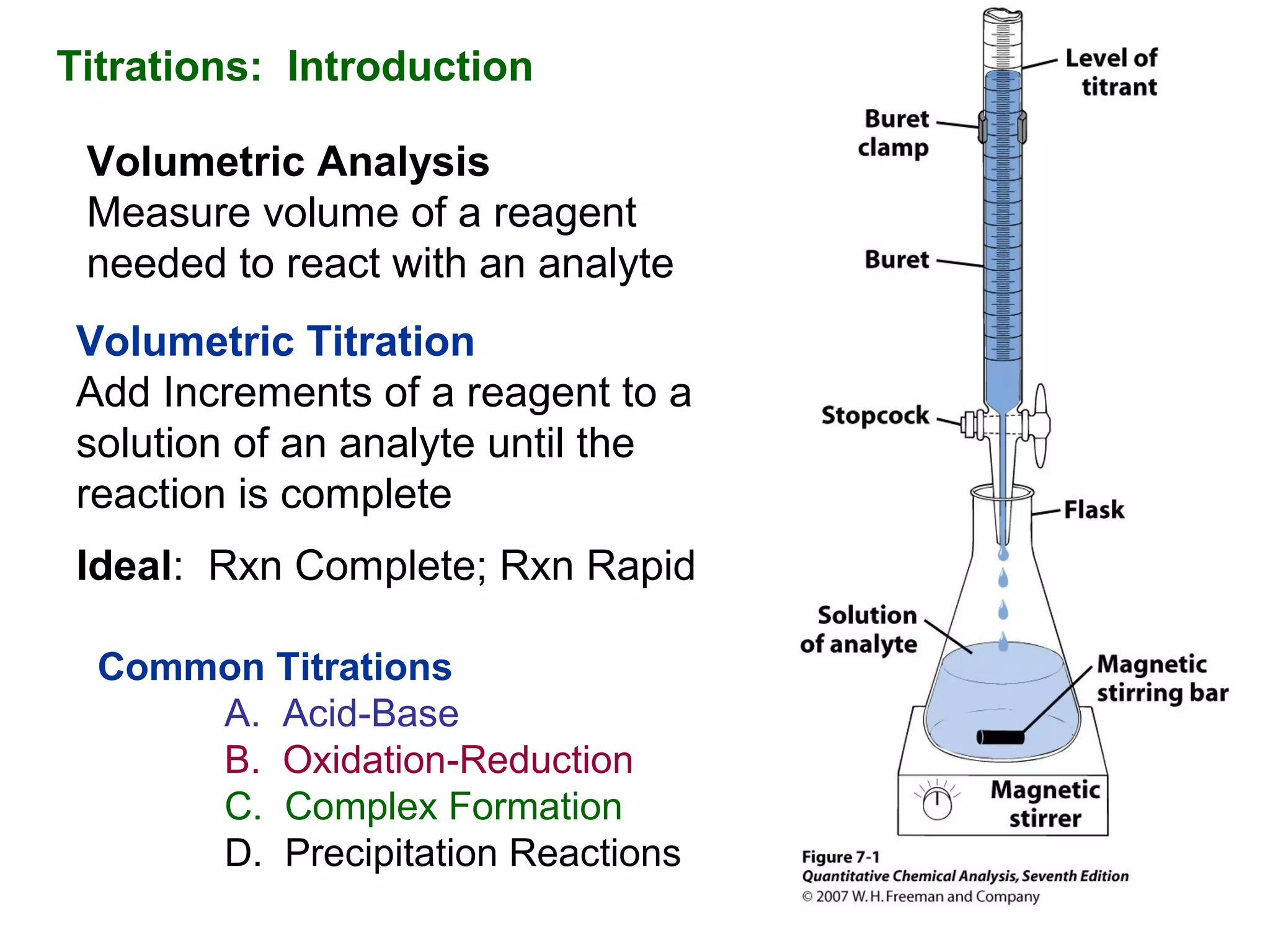
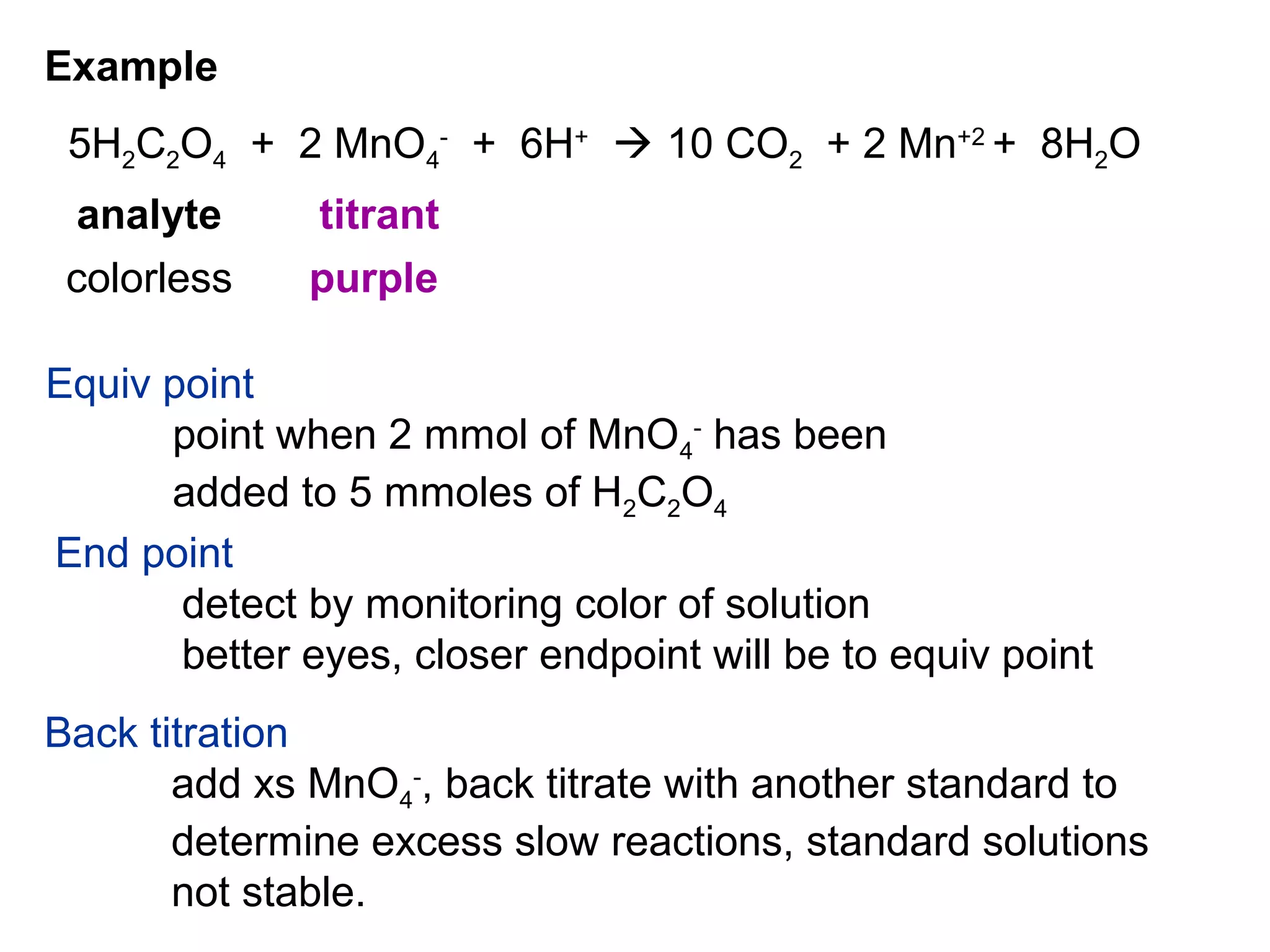
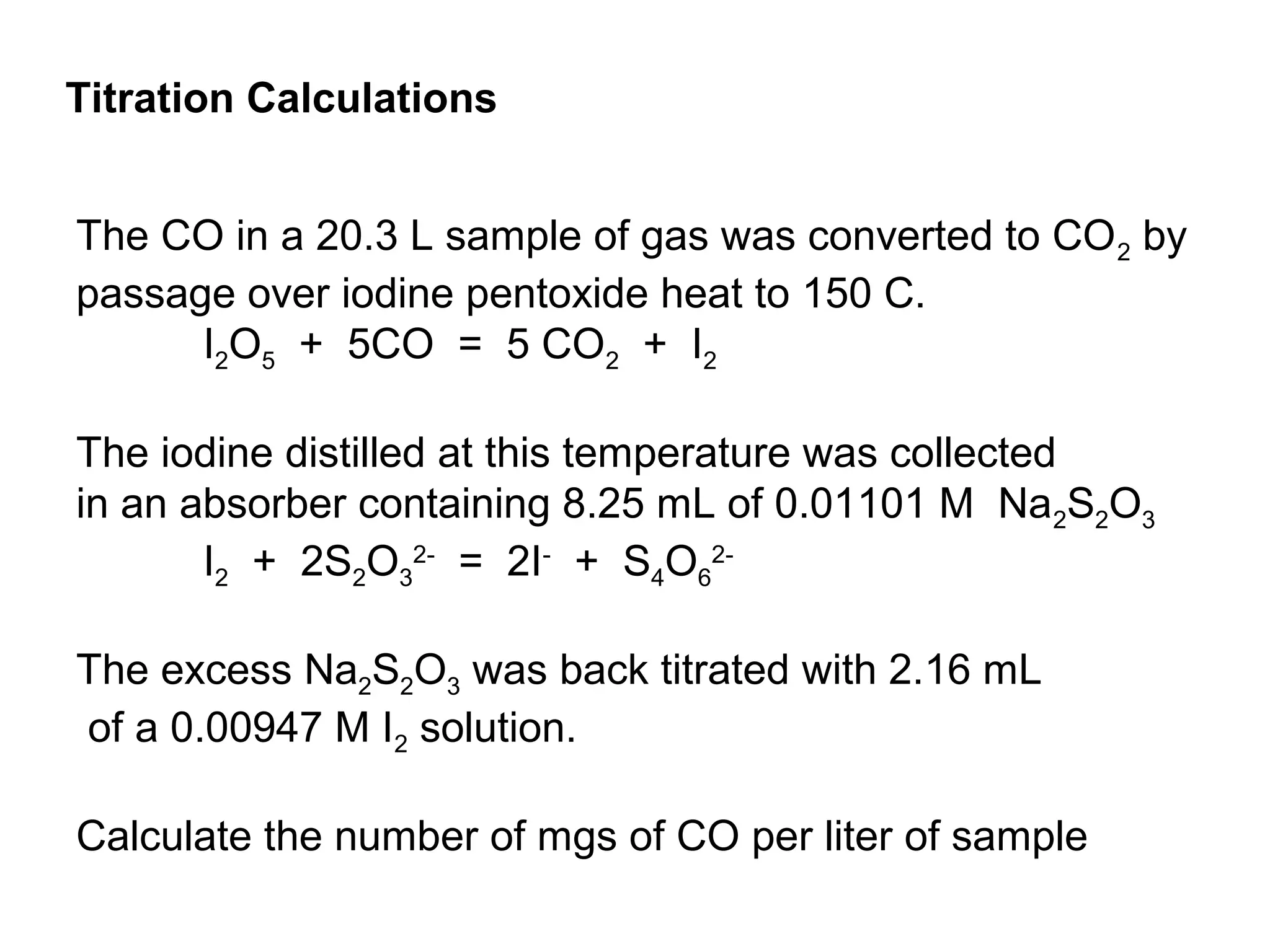
The document discusses different types of titrations including acid-base, oxidation-reduction, complex formation, and precipitation reactions. It defines key terms like indicator, equivalence point, and endpoint. Examples are provided for calculating concentration using titration data from reactions like acid-base titrations for chloride in urine and carbon monoxide determination. Steps are outlined for the Kjeldahl method to determine nitrogen content through acid digestion and titration.