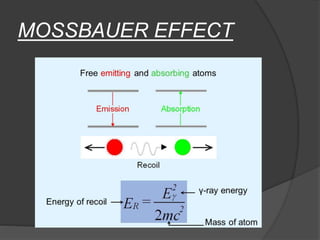
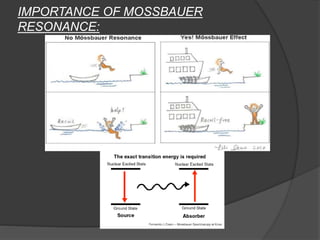
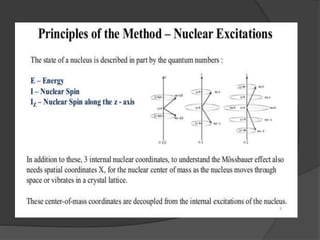
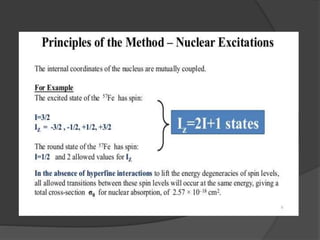
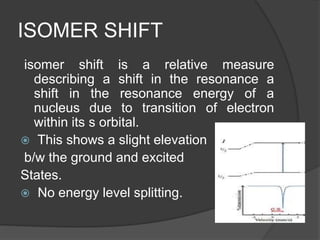
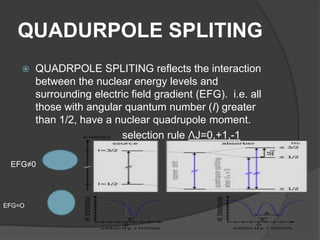
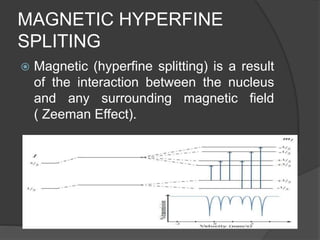
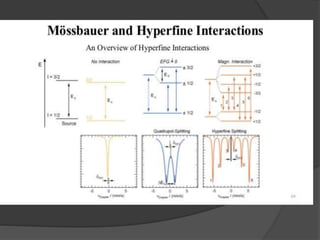
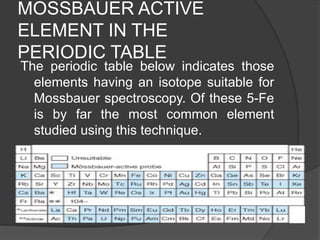
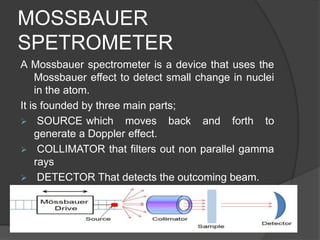
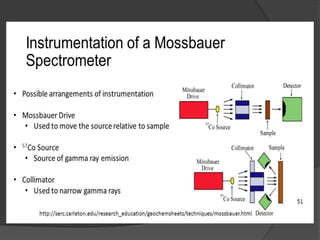
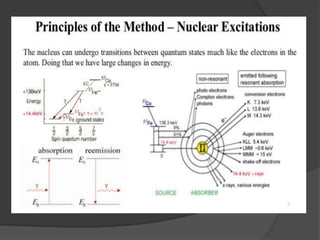
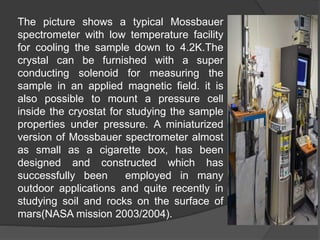
This document discusses the history and principles of Mössbauer spectroscopy, including the discovery of the Mössbauer effect by Rudolf Mössbauer in 1958 and its applications, notably in the Mars Exploration Rover missions. It explains key concepts such as resonance, isomer shift, quadrupole splitting, and magnetic hyperfine splitting while detailing the components and functionality of a Mössbauer spectrometer. The document highlights the significance of Mössbauer spectroscopy in analyzing materials, particularly iron-containing minerals, both on Earth and Mars.