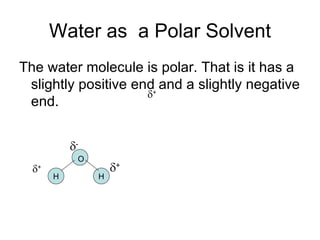
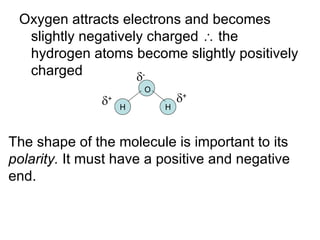
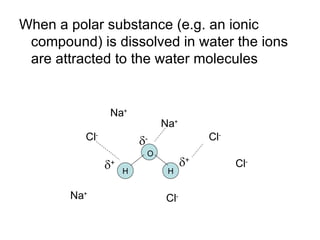
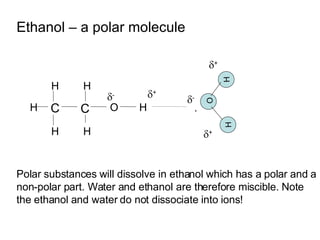
- Water is a polar solvent due to its molecular structure, with oxygen having a partial negative charge and hydrogen having partial positive charges. This allows it to dissolve ionic compounds by interacting with and separating the ions.
- Ionic compounds dissolve to varying degrees in water depending on how strongly the ions are attracted to each other versus water molecules. Solubility can be measured in g/L.
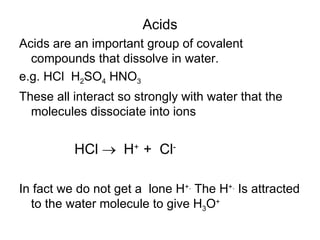
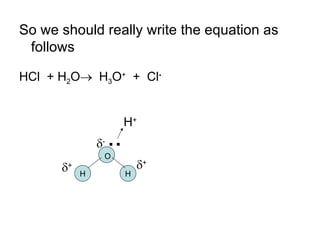
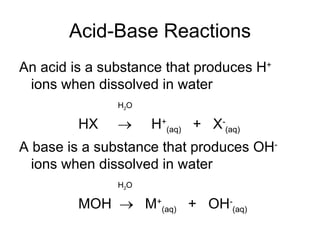
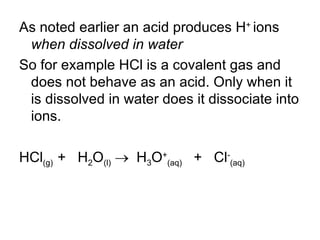
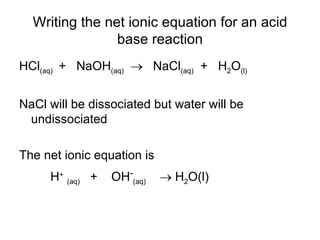
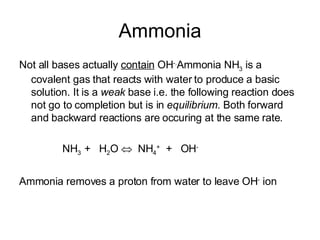
- Acids donate H+ ions in water and are classified as strong or weak based on how completely they ionize. Their strength affects pH calculations. Bases accept H+ and similarly ionize more or less completely.










![The pH scale pH is the negative log of H + concentration pH = - log [H + ] for a 0.1molar solution of HCl (a strong acid) pH = -log 0.1 = 1](https://image.slidesharecdn.com/9-aqueous-solutions2607/85/9-Aqueous-Solutions-23-320.jpg)
![What is the pH of a 0.01 mol solution of HCl? -log 0.01 = 2 What is the pH of a 0.1 molar solution of sulphuric acid? H 2 SO 4 2H + + SO 4 2- [H + ] = 2 x 0.1 = 0.2 mol/l And pH = -log 0.2 = 0.7](https://image.slidesharecdn.com/9-aqueous-solutions2607/85/9-Aqueous-Solutions-24-320.jpg)
![What is the pH of the following? 0.005 mol/l HNO 3 0.05 mol/l H 3 PO 4 [H + ] = 0.005 mol/L pH = -log 0.005 pH = 2.3 b) [H + ] = 3 x 0.05 mol/L pH = -log 0.15 pH = 0.82](https://image.slidesharecdn.com/9-aqueous-solutions2607/85/9-Aqueous-Solutions-25-320.jpg)
![Finding pH from [H + ] What is the [H + ] of a solution of HCl with a pH of 3? Antilog -3 = 0.001 [H] = 0.001 What is the [HCl]? From the equation HCl H + + Cl - we can see that the ratio of [H + ] to [HCl] = 1:1 [HCl] = 0.001 mol/L](https://image.slidesharecdn.com/9-aqueous-solutions2607/85/9-Aqueous-Solutions-26-320.jpg)
![What is the [H + ] of a solution of H 2 SO 4 with a pH of 2.4? Antilog -2.4 = 0.004 mol/L [H+] = 0.004 mol/L H 2 SO 4 2H+ + SO 4 2- [H 2 SO 4 ] = 0.004/2 = 0.002 mol/L What is the [H 2 SO 4 ]?](https://image.slidesharecdn.com/9-aqueous-solutions2607/85/9-Aqueous-Solutions-27-320.jpg)
![Finding pH from [OH - ] pOH = -log[OH - ] and pH +pOH = 14 What is the pH of a 0.1 mol/L solution of sodium hydroxide? pOH = -log 0.1 = 1 pH = 14 – 1 = 13](https://image.slidesharecdn.com/9-aqueous-solutions2607/85/9-Aqueous-Solutions-28-320.jpg)
![Find pH of the following solutions 0.001 mol/l potassium hydroxide 0.05 mol/L calcium hydroxide a) pOH = -log 0.001 = 3 pH = 14 -3 = 11 b) [OH - ] = 2 x 0.05 pOH = -log 0.1 = 1 pH = 14 -1 = 13](https://image.slidesharecdn.com/9-aqueous-solutions2607/85/9-Aqueous-Solutions-29-320.jpg)
![Calculate the pH of of a solution made by dissolving 1.00g of calcium hydroxide in 500ml of water. RMM Ca(OH) 2 = 40 + (2 x 16) + (2 x 1) = 74 1.00g = 1.00 = 0.0135mol 74 Moles [OH - ] = 2 x 0.0135 = 0.027 Moles per litre = 2 x 0.027 = 0.054 pOH = -log 0.054 = 1.27 pH = 14 – 1.27 = 12.3](https://image.slidesharecdn.com/9-aqueous-solutions2607/85/9-Aqueous-Solutions-30-320.jpg)