A document discusses various topics relating to chemistry solutions including:
1) The definition of solutions, solvents, and solutes. A solution is a homogeneous mixture of substances where the solute is the smaller component dissolved in the solvent.
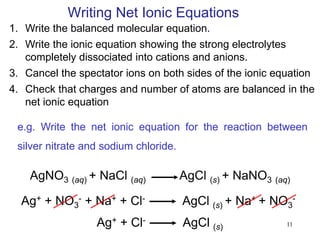
2) Properties of aqueous solutions including that electrolytes can conduct electricity while nonelectrolytes cannot. Strong electrolytes dissociate completely while weak electrolytes only partially dissociate.
3) Reactions involving solutions such as precipitation reactions, acid-base reactions, and redox reactions. Precipitation occurs when an insoluble solid forms. Acid-base reactions involve acids and bases reacting to form water and a salt. Redox reactions involve the transfer of electrons