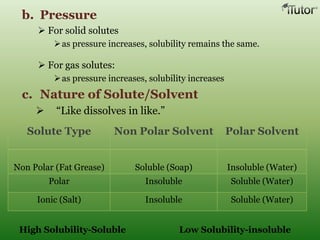
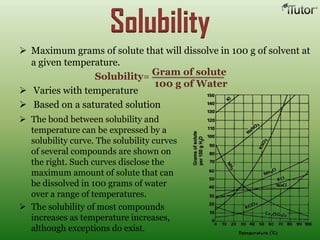
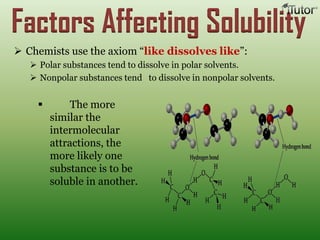
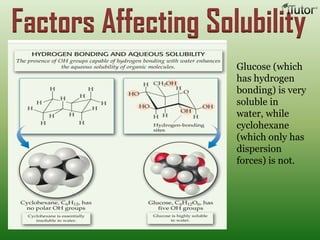
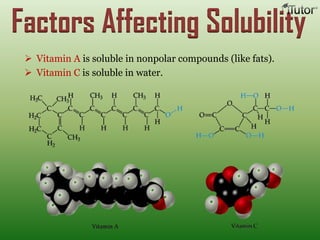
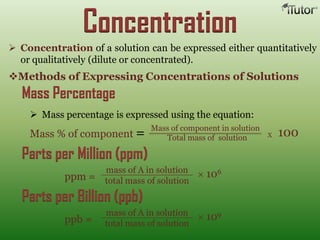
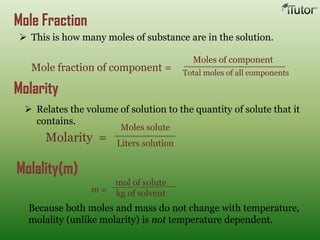
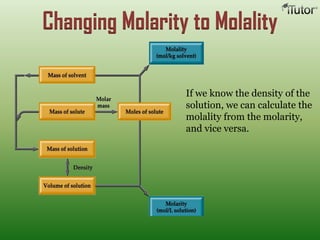
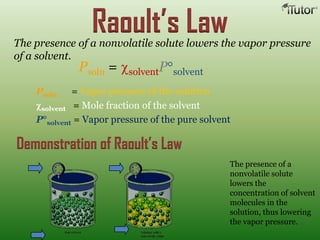
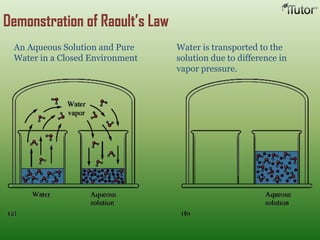
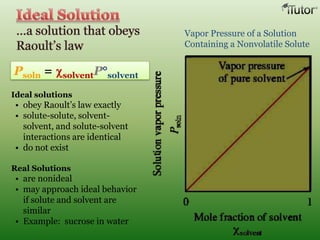
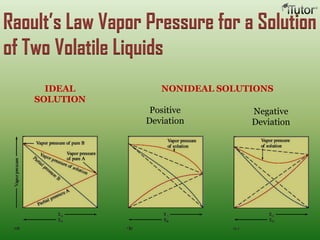
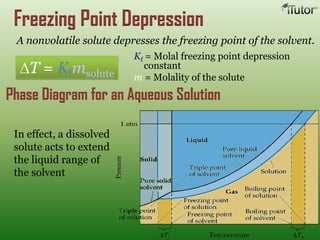
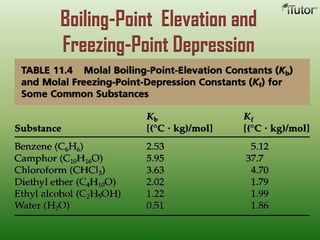
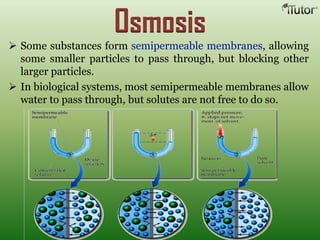
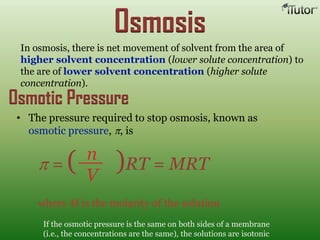
A solution is a homogeneous mixture of two or more substances, where the solute is dispersed uniformly throughout the solvent. The solubility of a solute is dependent on temperature, pressure, and the nature of the solute and solvent. Solubility is expressed as the maximum grams of solute that will dissolve per 100 grams of solvent. Colligative properties, such as boiling point elevation and freezing point depression, depend only on the number of solute particles and not their identity.