1) Equilibria occur in closed systems when the properties of the system no longer change with time, such as when the rates of the forward and reverse reactions are equal.
2) According to Le Châtelier's principle, if a stress is applied to a system at equilibrium, the equilibrium position will shift in a way that reduces the effect of the stress.
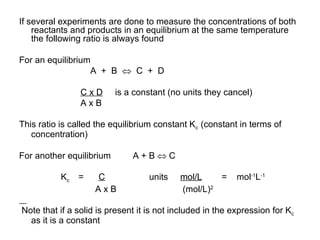
3) The equilibrium constant Kc is the ratio of products over reactants concentrations or partial pressures at equilibrium. If Kc is greater than 1, more products form, and if less than 1, more reactants form.





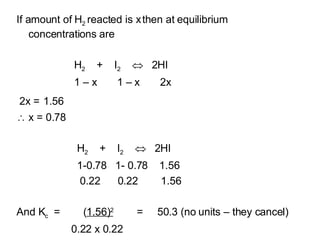
![A mixture of 1 mol hydrogen and 1 mol iodine are reacted together and reach equilibrium at constant temperature 1.56 mols of HI are present at equilibrium. Find K c H 2 + I 2 2HI K c = [ HI] x [HI] = [HI] 2 [H 2 ] x [I 2 ] [H 2 ][I 2 ]](https://image.slidesharecdn.com/7-equilibria4272/85/7-Equilibria-7-320.jpg)