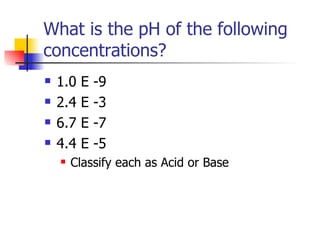
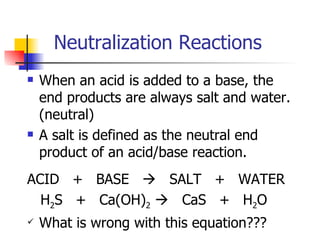
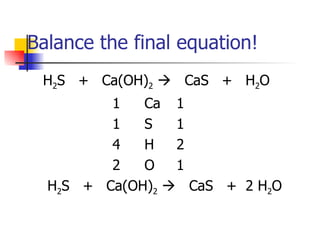
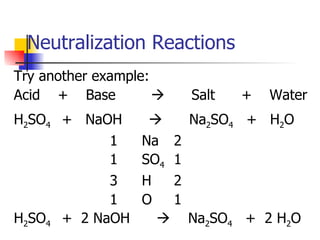
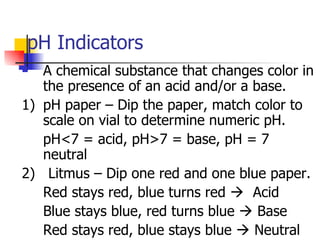
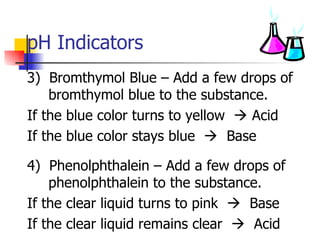
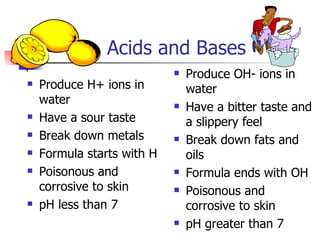
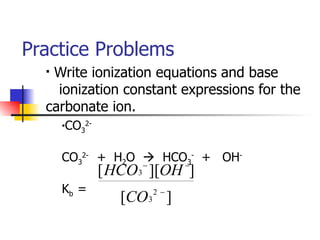
This document provides information about acids and bases including:
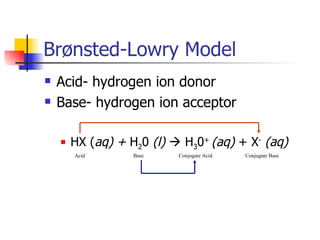
1) Common acids and their names, acid-base definitions using Arrhenius and Brønsted-Lowry models, and properties of acids and bases.
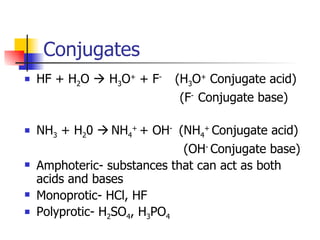
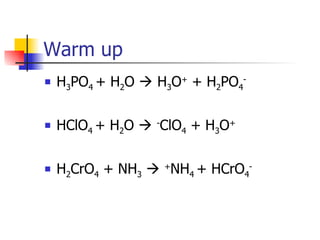
2) Conjugate acid-base pairs, acid and base strength, pH and pOH calculations.
3) Buffers, neutralization reactions, and examples of pH indicators.


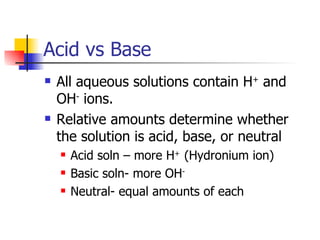
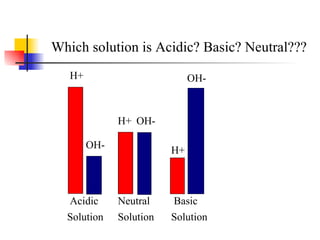




![pH Measure of H + ions in soln pH = -log[H + ] Acidic solutions have a pH below 7 Basic solutions have a pH above 7 pH 7 is neutral Change of 1 pH unit represents a tenfold change. (exponential)](https://image.slidesharecdn.com/acidbasenotesh-100114105228-phpapp01/85/Acid-Base-Notes-H-18-320.jpg)
![pOH Measures concentration of OH - ion pOH = - log [OH - ] pH + pOH = 14.00](https://image.slidesharecdn.com/acidbasenotesh-100114105228-phpapp01/85/Acid-Base-Notes-H-19-320.jpg)
![Practice Problems Calculate the pH and pOH of aqueous solutions having the following ion concentrations. [OH - ] = 6.5 x 10 -6 pOH = -log[OH - ] pH = 14.00 – pOH pOH = -log[6.5 x 10 -6 ] pH = 14.00 – 5.19 pOH = -[log 6.5 + log 10 -6 ] pH = 8.81 pOH = -[0.81 + (-6)] pOH = 5.19](https://image.slidesharecdn.com/acidbasenotesh-100114105228-phpapp01/85/Acid-Base-Notes-H-20-320.jpg)
![One more, one more time! Calculate the pH and pOH of aqueous solutions having the following ion concentrations. [H + ] = 3.6 x 10 -9 pH = -log[H + ] pOH=14.00 – pH pH = -log[3.6 x 10 -9 ] pOH=14.00-8.44 pH = -[log 3.6 + log 10 -9 ] pOH=5.56 pH = -[0.56 + (-9)] pH = 8.44](https://image.slidesharecdn.com/acidbasenotesh-100114105228-phpapp01/85/Acid-Base-Notes-H-21-320.jpg)