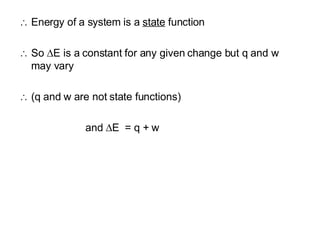
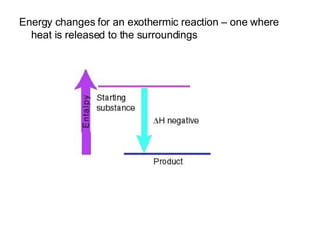
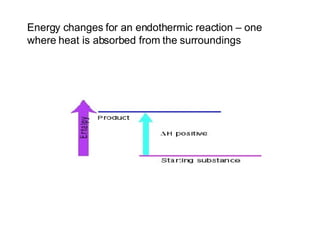
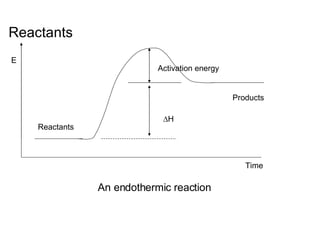
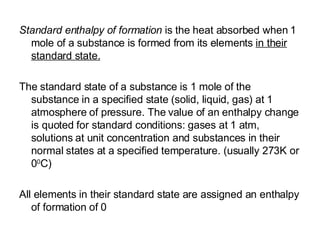
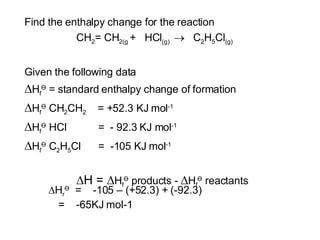
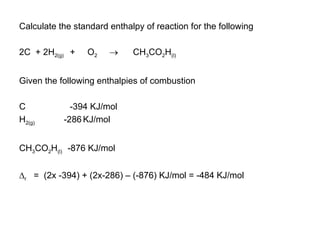
The document discusses key concepts in thermodynamics including:
1) The first law of thermodynamics states that energy cannot be created or destroyed, only changed from one form to another.
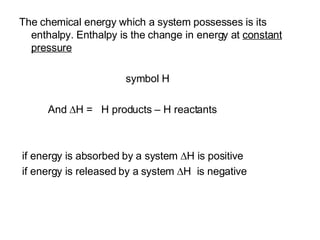
2) Enthalpy is a measurement of the total energy of a system at constant pressure.
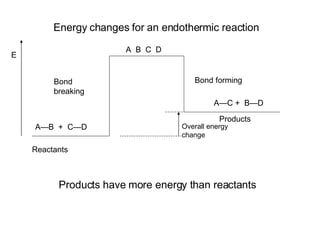
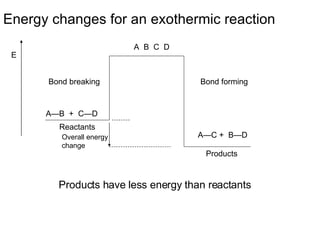
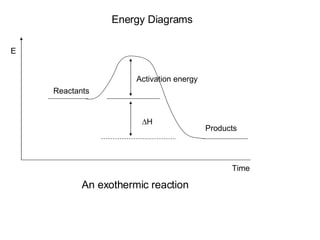
3) Chemical reactions can be exothermic or endothermic depending on whether energy is released or absorbed.