This document provides an introduction to acids and bases, including:
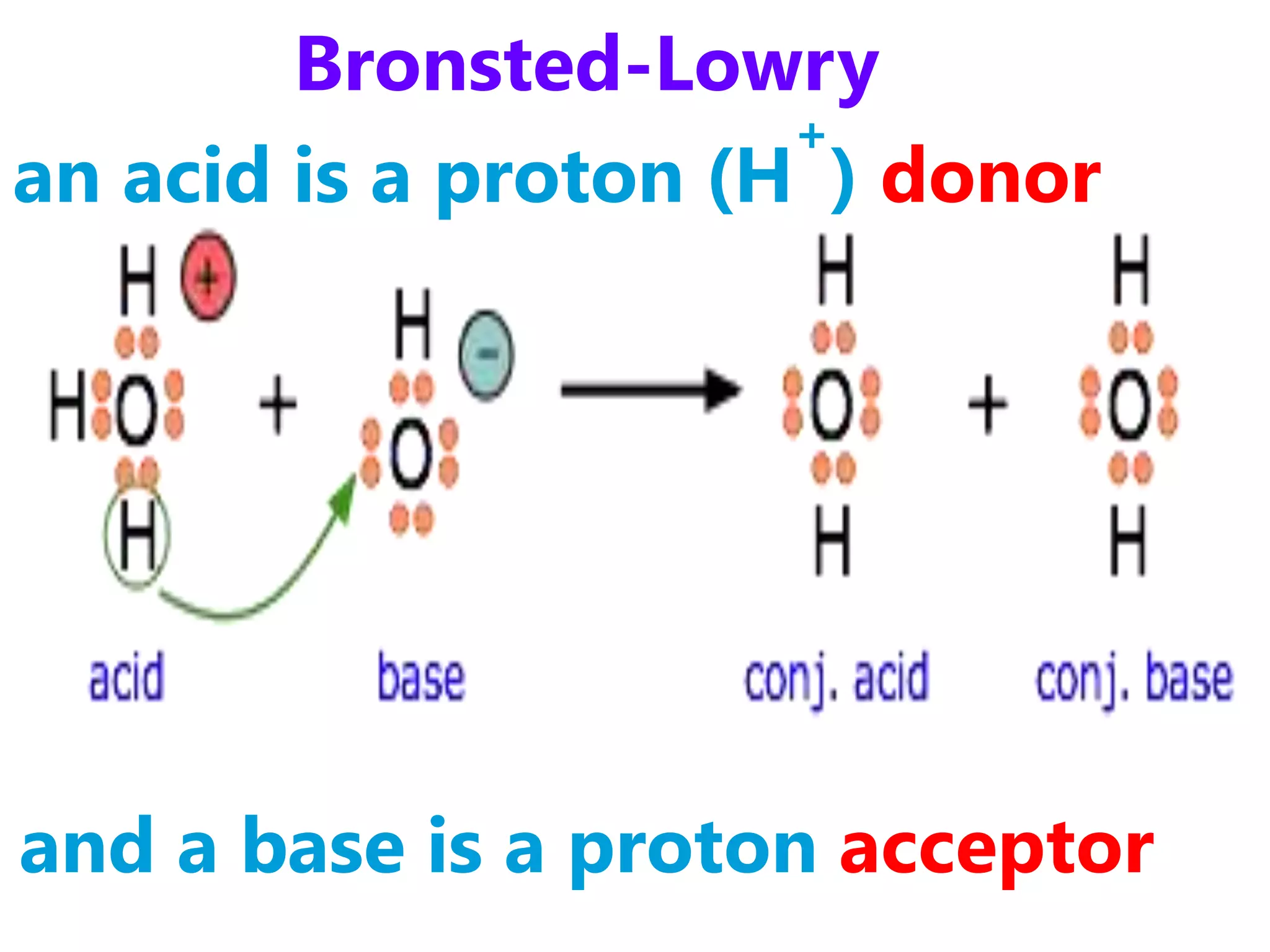
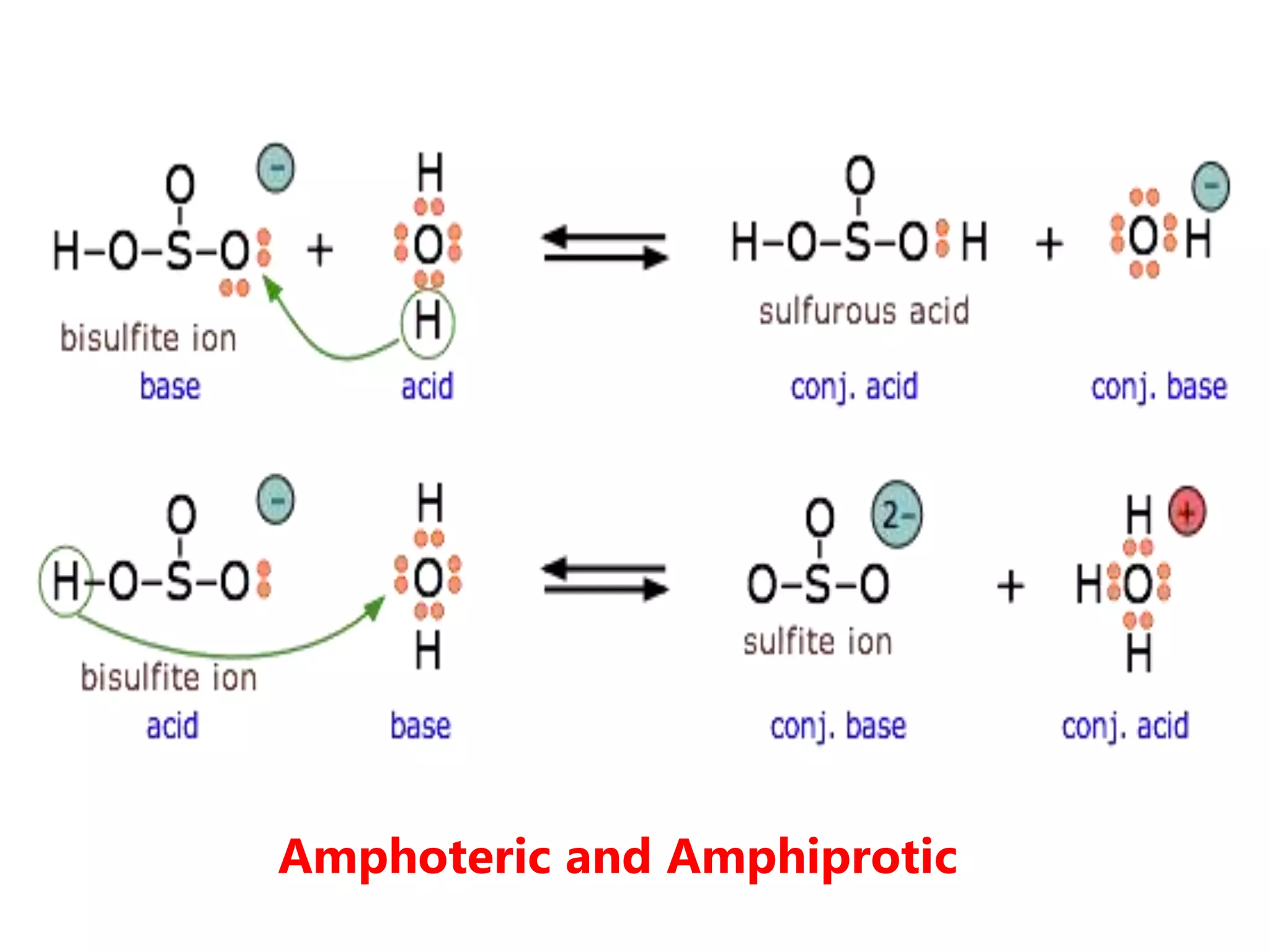
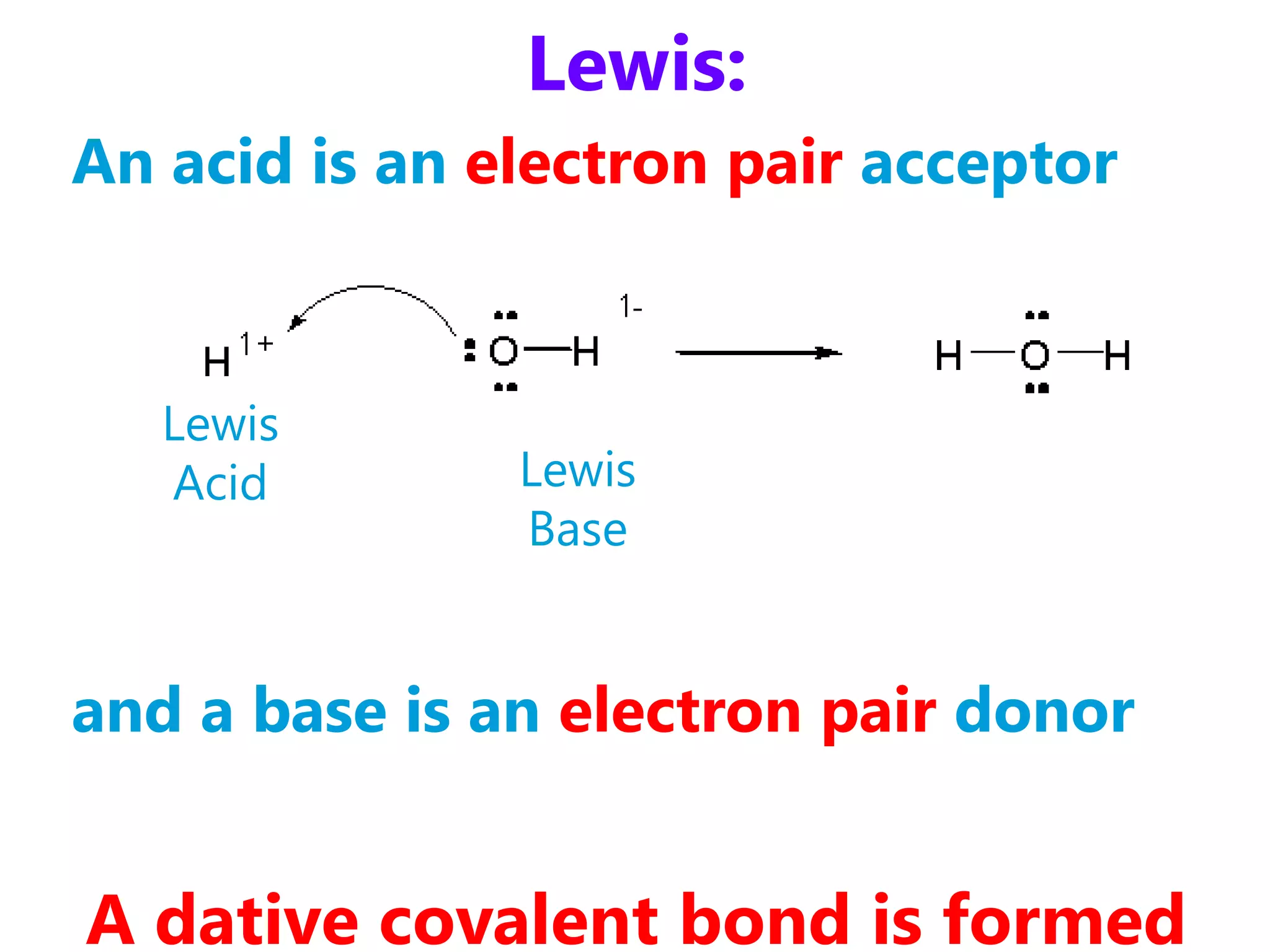
1) How acids and bases are defined according to the Arrhenius, Bronsted-Lowry, and Lewis theories. Acids donate protons while bases accept protons.
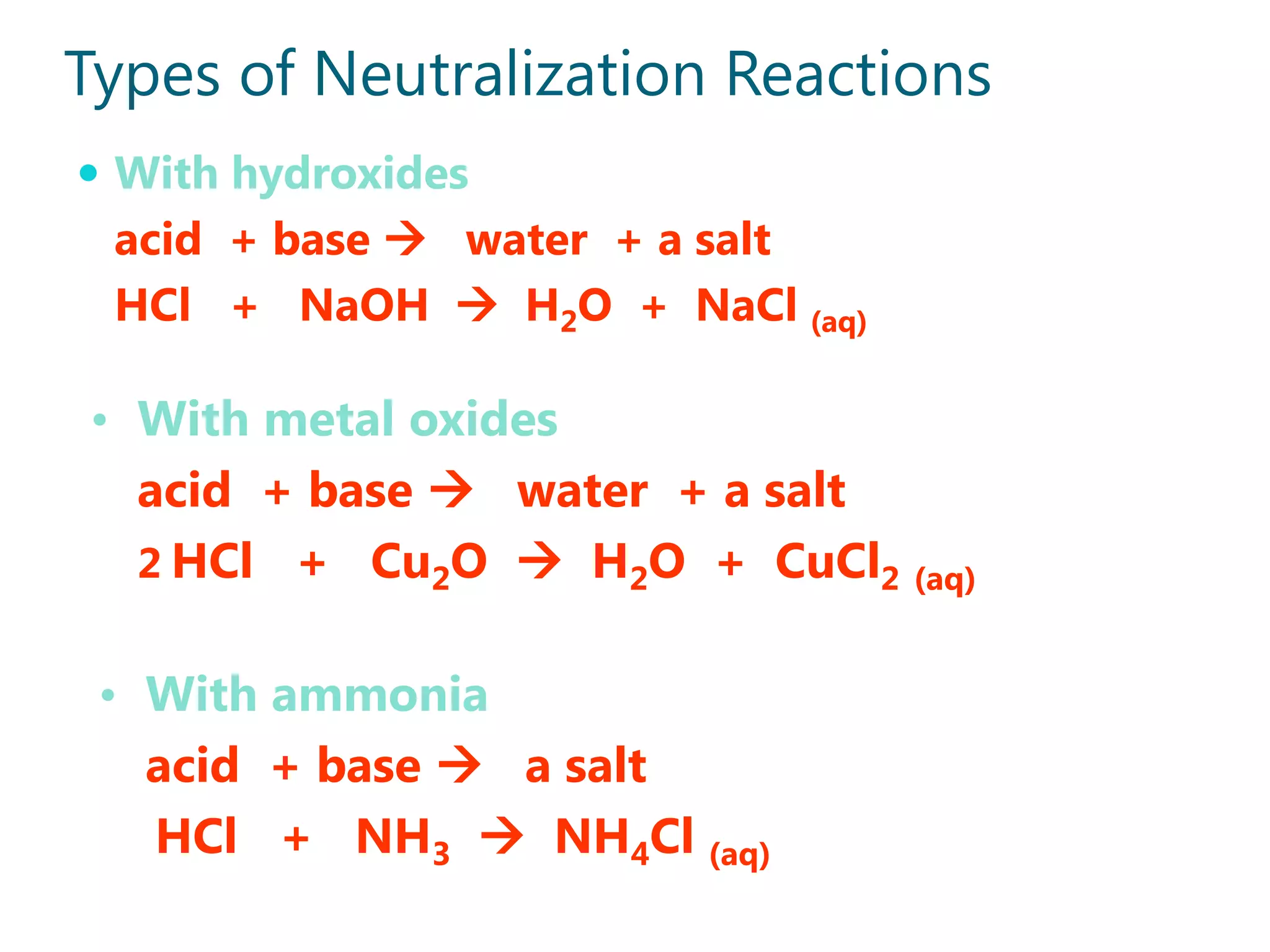
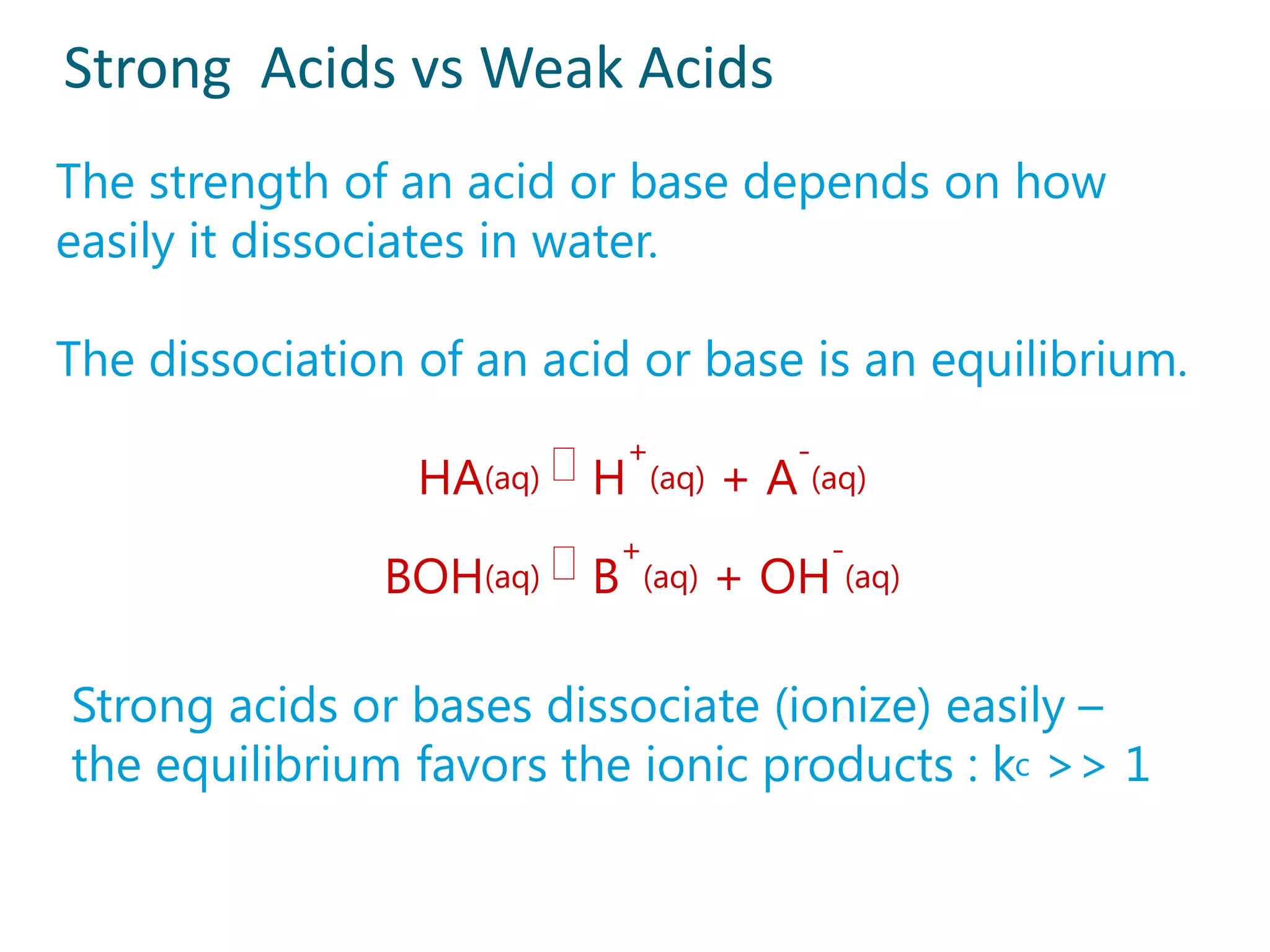
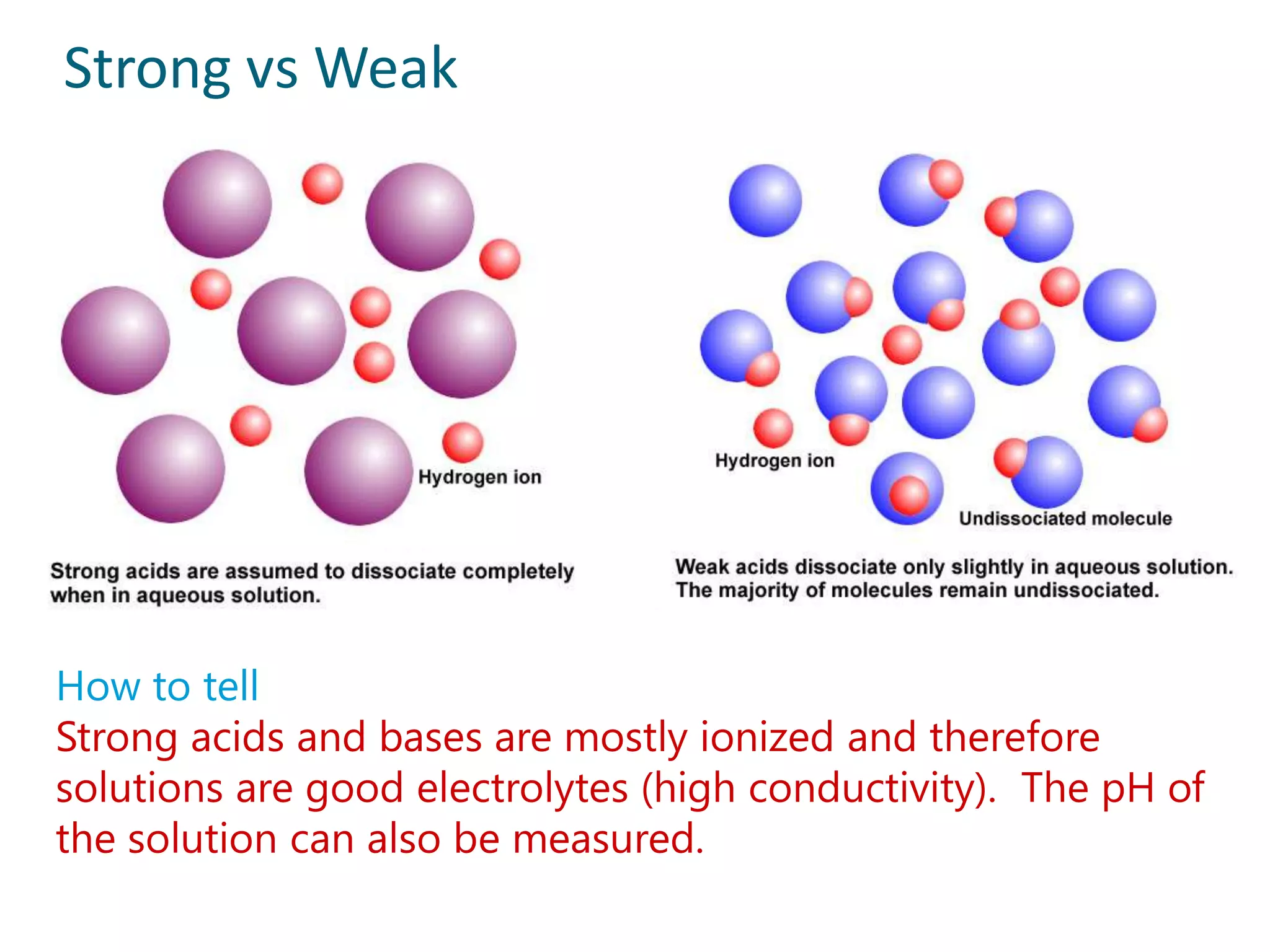
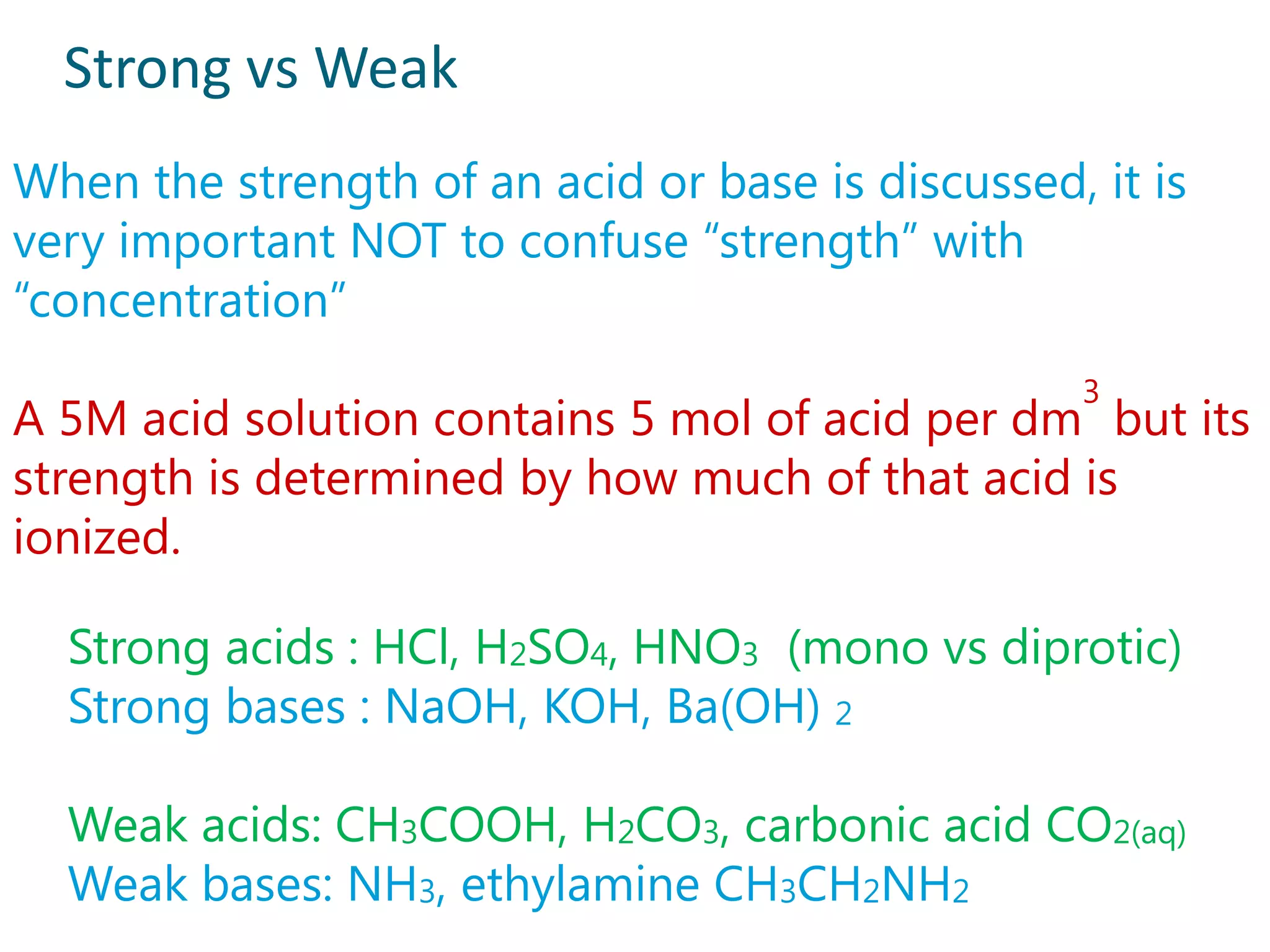
2) Examples of strong acids like HCl and weak acids like acetic acid. Strong acids fully dissociate in water while weak acids only partially dissociate.
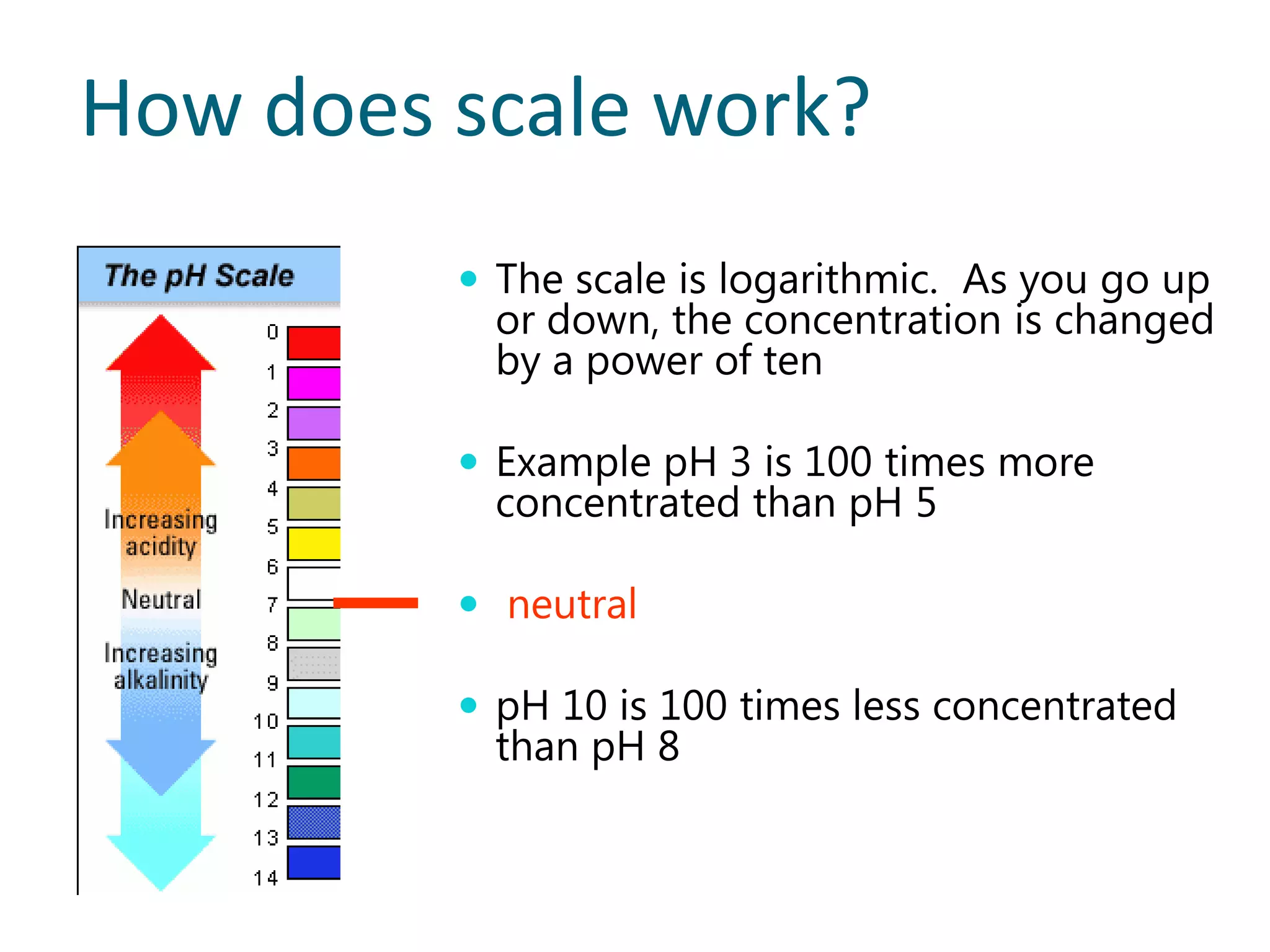
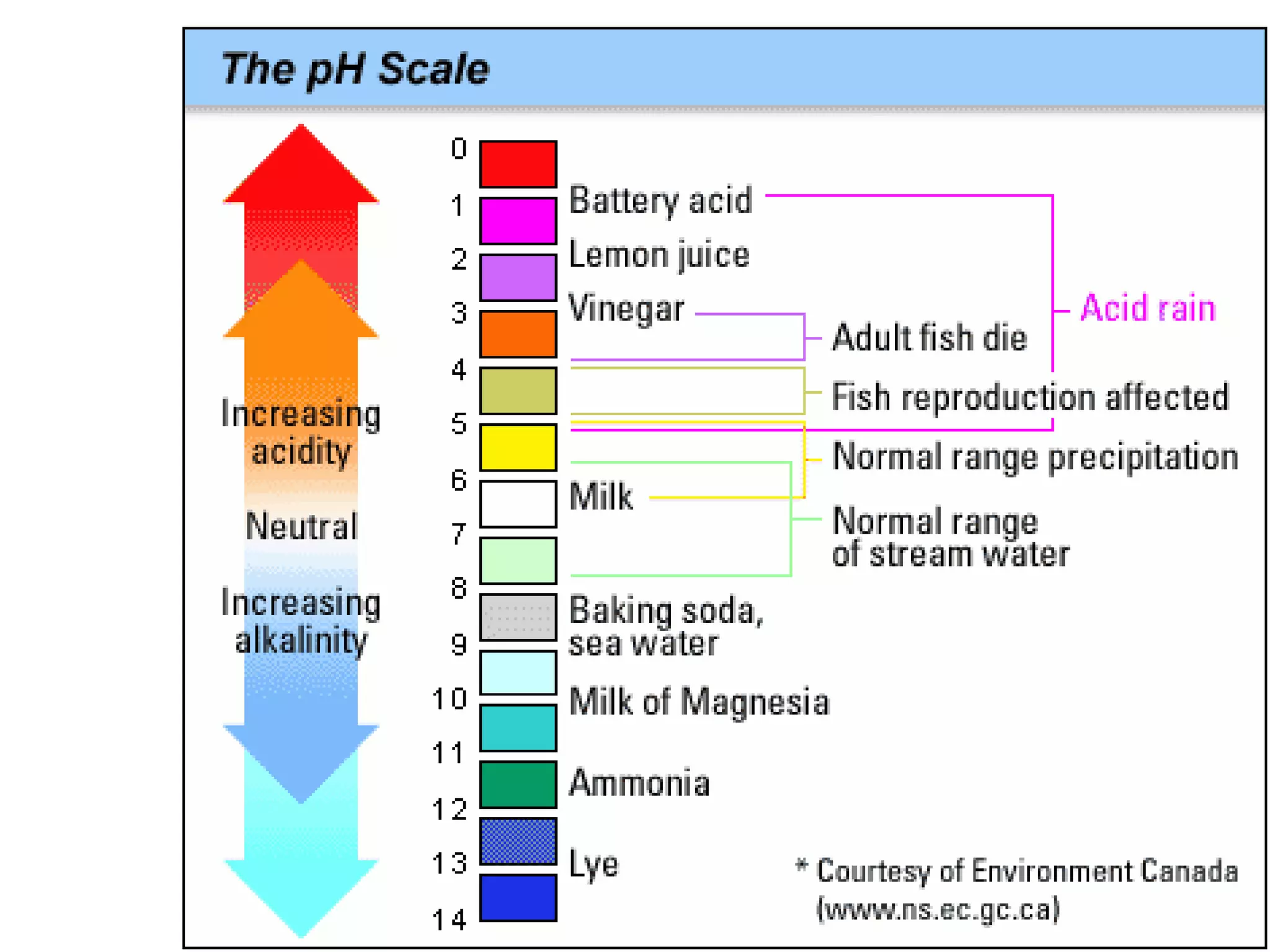
3) The pH scale measures hydrogen ion concentration from 0-14, with lower values being more acidic and higher more basic. Neutral solutions have a pH of 7.

![In aqueous solutions, a proportion of the water
molecules dissociate;
The ions formed are H+ or positively charged
hydrogen ions and negatively charged hydroxide ions
(OH-)
Technically
+ -
2 H2O(l) H3O (aq) + OH (aq)
+ − -14
Equilibrium Constant Kw = [H ][OH ] = 1 x 10](https://image.slidesharecdn.com/topic08-introduction-120905184045-phpapp02/75/Topic-08-introduction-2-2048.jpg)
![ Some chemical compounds contribute additional H+
to make the solution more acidic. Other compounds
remove H+ ions.
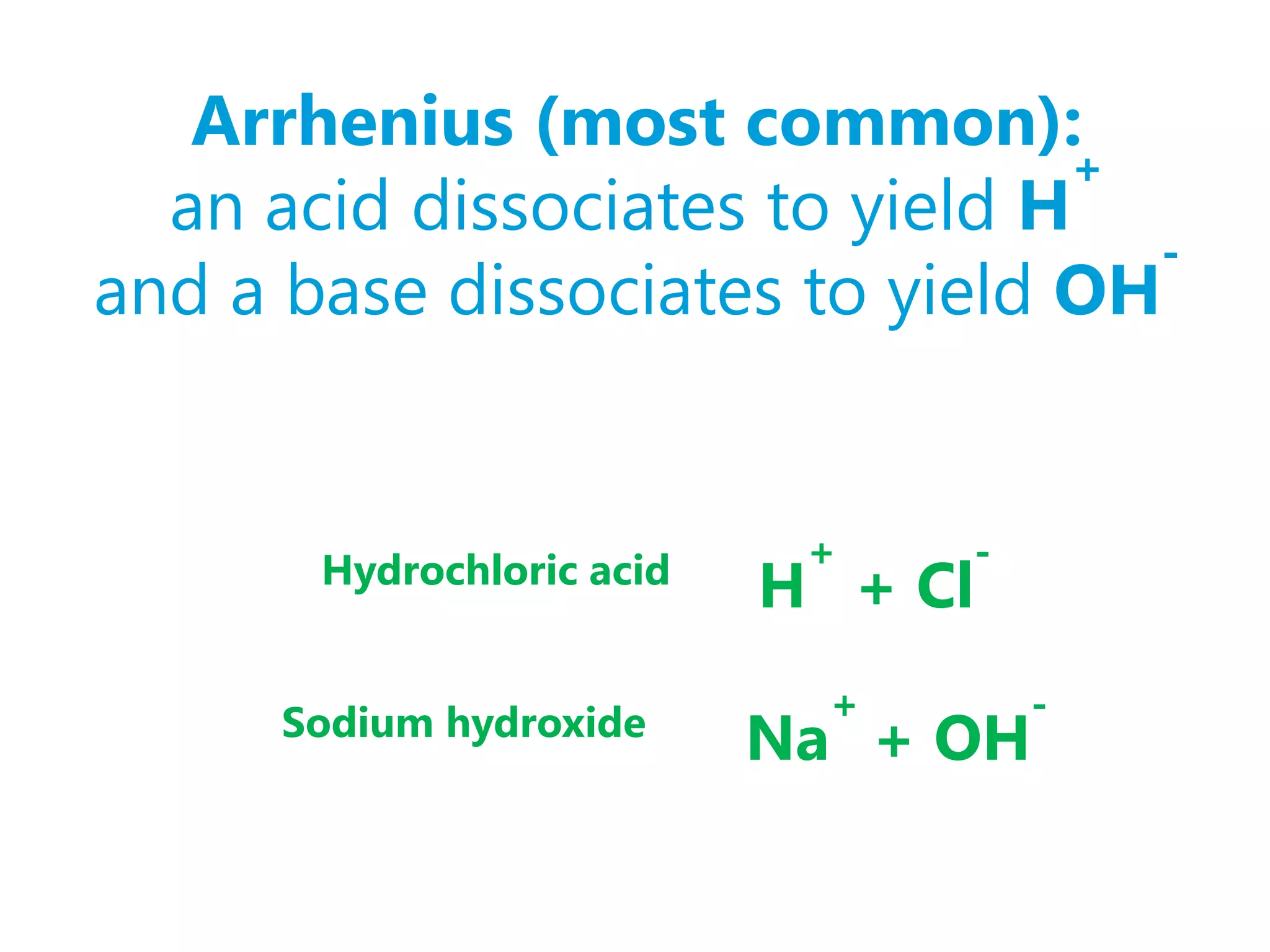
A compound that increases [H+] is called an acid
Examples: HCl, H2SO4, HNO3, CH3COOH](https://image.slidesharecdn.com/topic08-introduction-120905184045-phpapp02/75/Topic-08-introduction-3-2048.jpg)


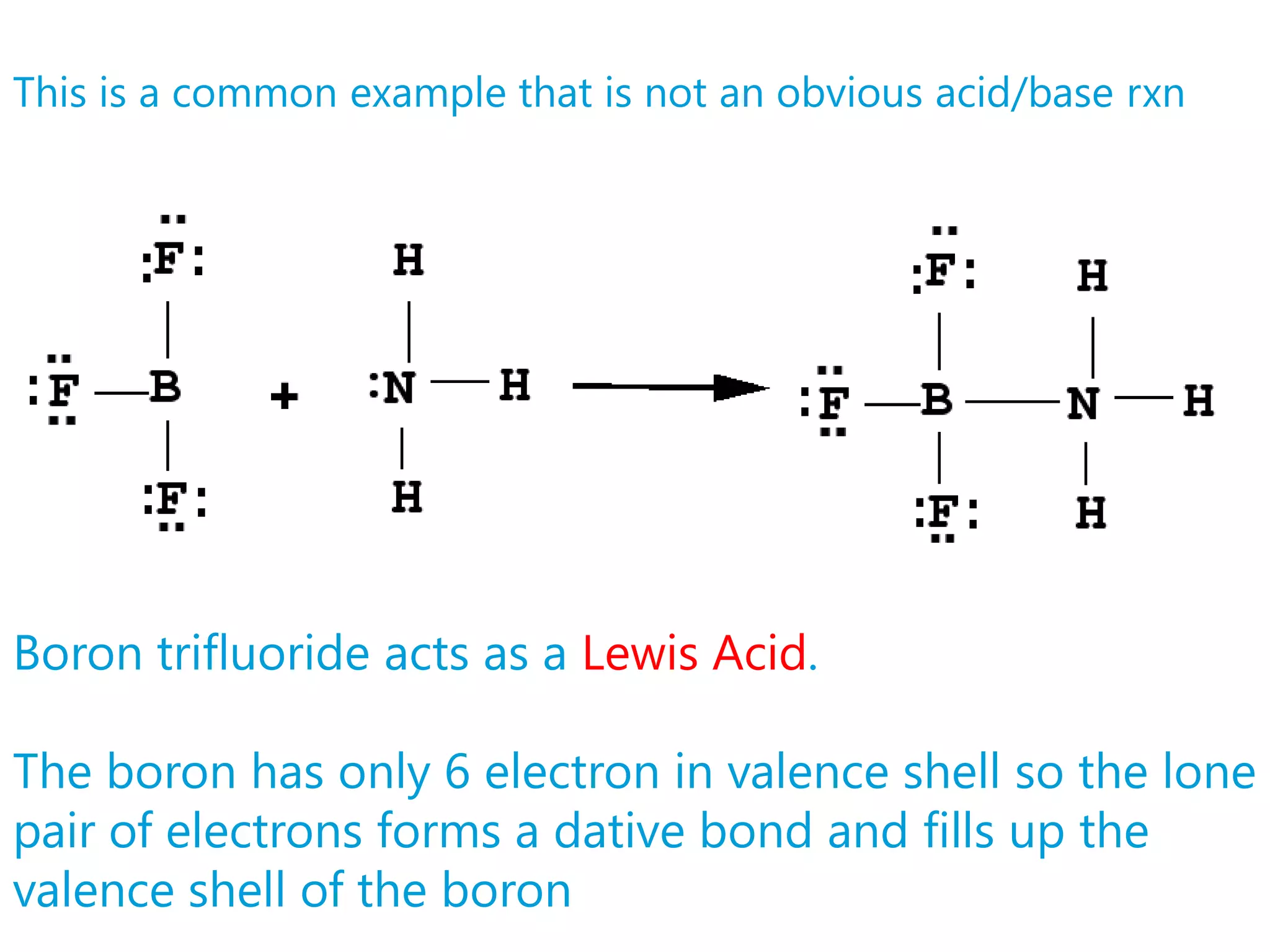

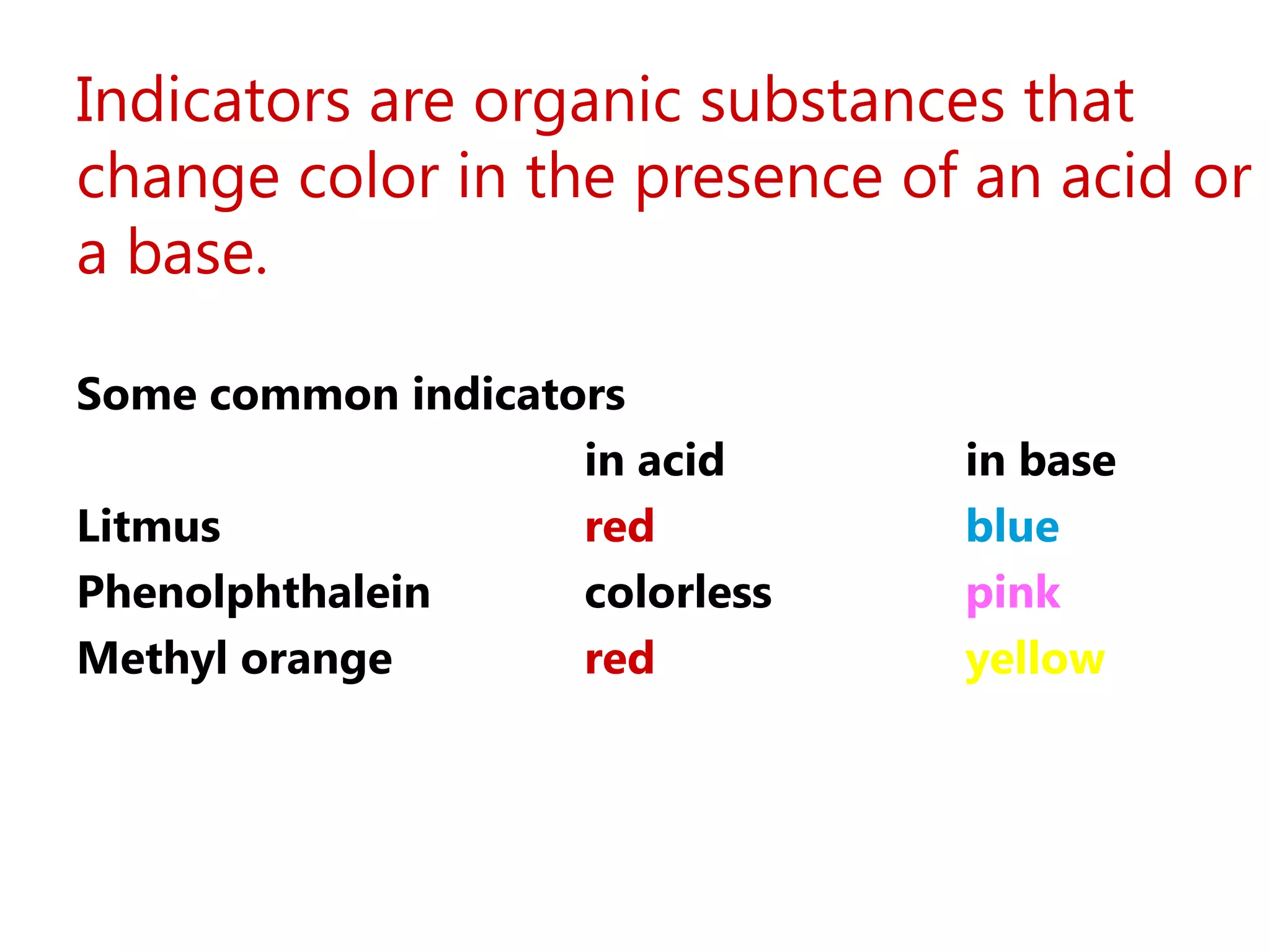
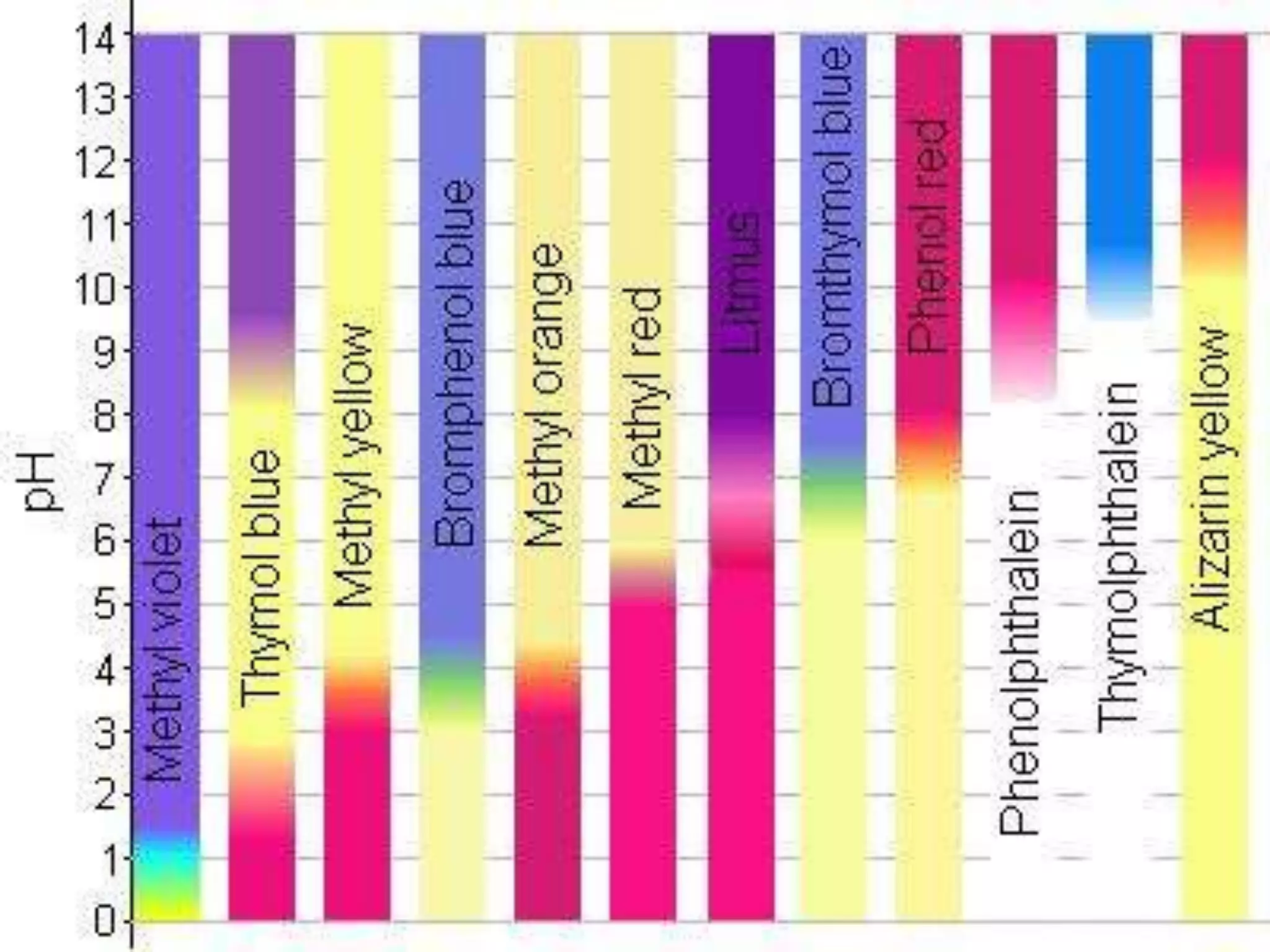
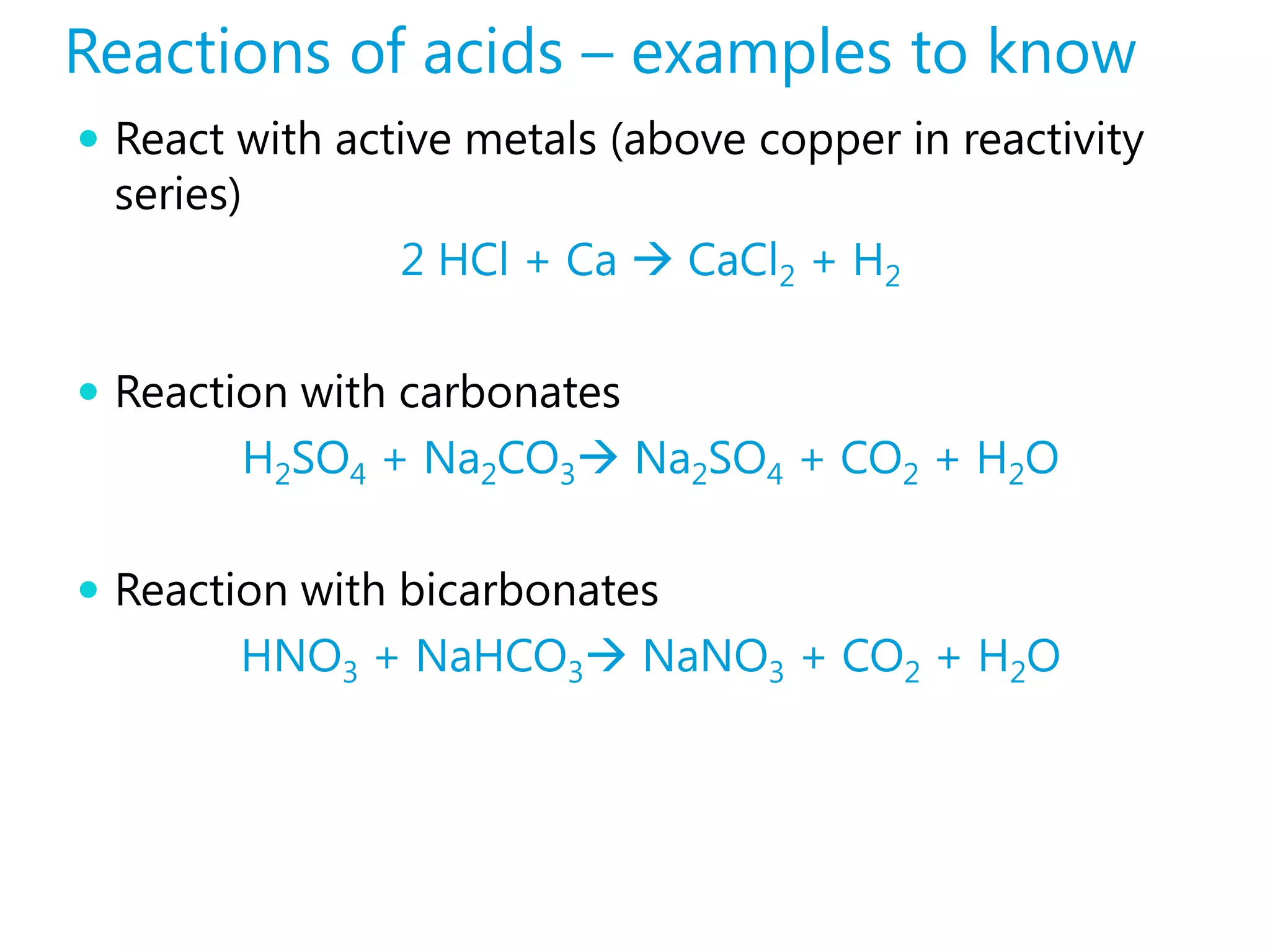

![Strong Acid
example HCl
+ -
HCl(aq) H (aq) + Cl (aq)
[H+ ][Cl- ]
k= >> 1
[HCl]
• completely dissociated
• pH of 0.1 M soln = 1
• strong electrolyte
• reacts vigorously
• note simplified “net ionic”
equation](https://image.slidesharecdn.com/topic08-introduction-120905184045-phpapp02/75/Topic-08-introduction-22-2048.jpg)
![Weak Acid
example CH3COOH
+ -
CH3COOH (aq) H (aq) + CH3COO (aq)
[H+ ][CH3COO- ]
k= << 1
[CH3COOH]
• partially dissociated
• pH of 0.1 M soln = 2.9
• weak electrolyte
• reacts slowly](https://image.slidesharecdn.com/topic08-introduction-120905184045-phpapp02/75/Topic-08-introduction-23-2048.jpg)
![What is the pH scale?
pH is a measurement of hydrogen ion concentration
It tells you how acidic or basic (or alkaline) something is
Ranges from 0 (most acidic) to 14 (most basic)
pH log[ H ]](https://image.slidesharecdn.com/topic08-introduction-120905184045-phpapp02/75/Topic-08-introduction-24-2048.jpg)