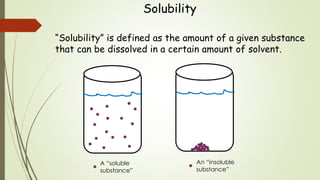
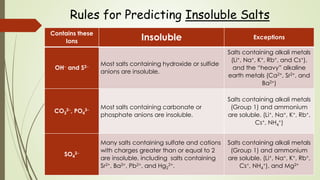
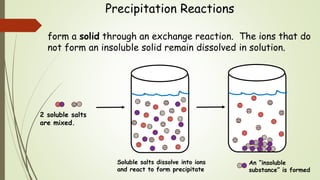
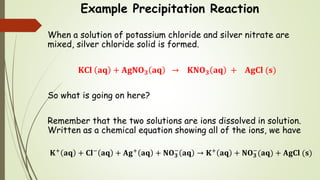
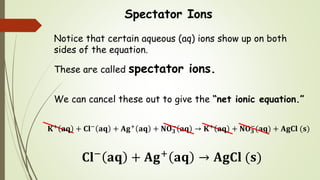
This document discusses the concept of solubility, categorizing substances into soluble, sparingly soluble, and insoluble based on their ability to dissolve in solvent. It provides rules for predicting the solubility of salts with examples of precipitation reactions and explains how to identify spectator ions and write net ionic equations. The key takeaway is the ability to determine soluble and insoluble compounds, predict precipitates in reactions, and formulate net ionic equations.