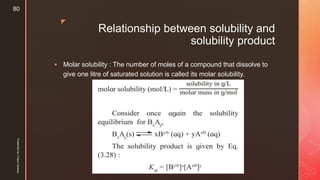
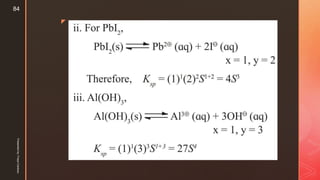
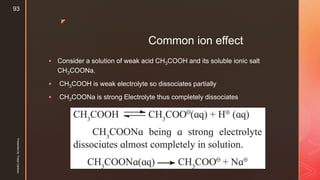
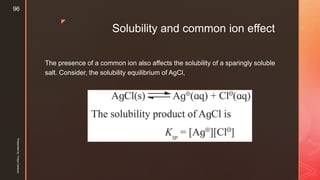
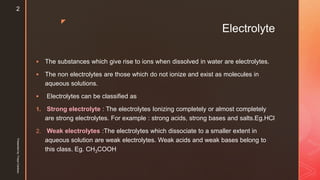
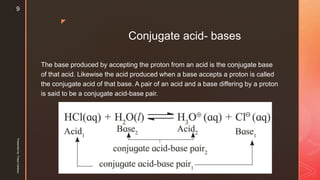
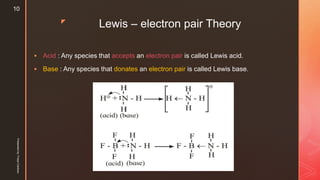
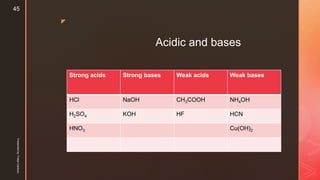
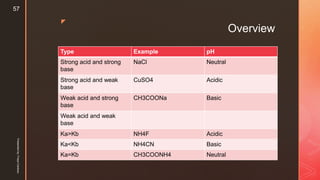
The document discusses ionic equilibrium and acid-base theories. It provides examples of different types of salts based on the strength of acids and bases involved:
1) Salts of strong acids and bases, like NaCl, are neutral as they do not undergo hydrolysis.
2) Salts of strong acids and weak bases, like CuSO4, are acidic due to hydrolysis of the metal cation.
3) Salts of weak acids and strong bases, like CH3COONa, are basic due to hydrolysis of the anion.
4) Salts of weak acids and weak bases can be acidic, basic or neutral depending on whether the Ka or Kb is greater and the extent of hydro











![z
Calculate [H3O⊕] in 0.1 mol dm3 solution of acetic acid.
Given : Ka [CH3COOH] = 1.8 × 10-5
Presentedby:FreyaCardozo
27](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-27-320.jpg)

![z
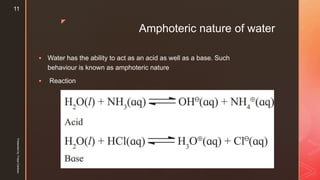
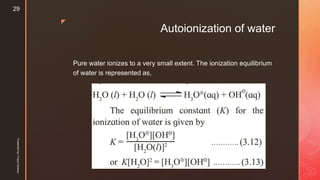
A majority of H2O molecules are undissociated, consequently concentration of water
[H2O] can be treated as constant
Kw = KK' is called ionic product of water. The product of molar concentrations of
hydronium (or hydrogen) ions and hydroxyl ions at equilibrium in pure water at the
given temperature is called ionic product of water.
Presentedby:FreyaCardozo
30](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-30-320.jpg)

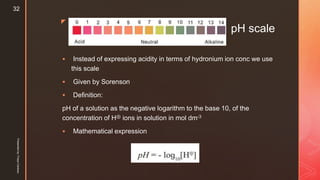
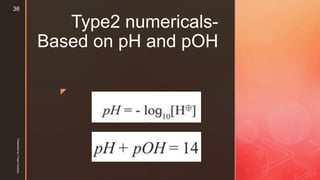
![z
pOH of a solution can be defined as the negative logarithm to the
base 10, of the molar concentration of OH ions in solution.
Thus, pOH = -log10[OH- ]
Presentedby:FreyaCardozo
33](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-33-320.jpg)

![z
Types of solutions based on pH
Neutral solutions
For pure water or any aqueous neutral solution at 298 K
[H3O⊕] = [OH -] = 1.0 × 10-7 M
Hence, pH = -log10[H⊕] = -log10[1 × 10-7] = 7
Acidic solutions
In acidic solution, there is
Excess of H3O⊕ ions, or [H3O⊕] > [OH ] Hence, [H3O⊕] > 1 × 10-7 and pH < 7
Basic solutions
In basic solution, the excess of OH ions are present that is [H3O⊕] < [OH ] or
[H3O⊕] < 1.0 × 10-7 with pH > 7.
Presentedby:FreyaCardozo
35](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-35-320.jpg)

![z
pH of a solution is 3.12.
Calculate the concentration of H3O⊕ ion.
pH= -log[H+]
-3.12= log[H+]
-3-0.12+1-1= log[H+]
-4+0.88= log[H+]
-4.88= log[H+]
[H+]=7.586 x 10-4
Presentedby:FreyaCardozo
38](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-38-320.jpg)
![z
A weak monobasic acid is
0.04 % dissociated in 0.025M solution.
What is pH of the solution ?
Given : alpha= 0.04/100
c=0.025M
To find: pH=?
[H+]= alpha x c= 0.0004x 0.025= 10-5
pH=5
Presentedby:FreyaCardozo
39](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-39-320.jpg)



![z
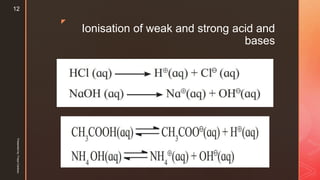
Hydrolysis of water
When a salt is dissociated in water, it dissociates completely into its constituent
ions.
The solvent water dissociates slightly as,
Pure water is neutral and [H3O⊕] = [OH -].
If the ions of the salt do not interact with water, the hydronium and hydroxyl ion
concentrations remain equal and the solution is neutral
Presentedby:FreyaCardozo
43](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-43-320.jpg)














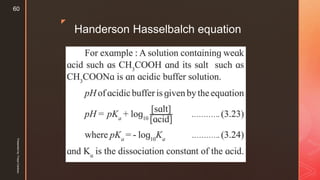

![z
If a small amount of strong acid is added to this solution the added H⊕ ions will be
consumed by the conjugate base CH3COO- present in large concentration.
if small amount of base is added, the added OH ions will be neutralized by the large
concentration of acetic acid
The acid or base added thus can not change the [H⊕] or [OH ] concentrations and, pH
of the buffer remains unchanged.
Presentedby:FreyaCardozo
63](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-63-320.jpg)



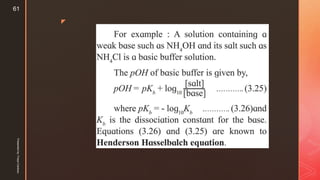
![z
Type 3 numericals
Based on Henderson-Hasselbach equation
Ka/Kb Dissociation constant for acid / base
pKa= -logKa
[salt] concentration of salt
[acid]/[base]: Tip- To know if it is salt or acid see which has H+
Presentedby:FreyaCardozo
67](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-67-320.jpg)
![z
Type 3 Numericals
Calculate the pH of buffer solution containing 0.05 mol NaF per litre
and 0.015 mol HF per litre. [Ka = 7.2 × 10-4for HF]
Presentedby:FreyaCardozo
68](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-68-320.jpg)
![z
Calculate the pH of buffer solution composed of 0.1 M weak base BOH
and 0.2 M of its salt BA. [Kb = 1.8× 10-5 for the weak base]
Presentedby:FreyaCardozo
69](https://image.slidesharecdn.com/3-200905163255/85/Ionic-equilibrium-chapter-3-12th-HSC-Maharashtra-state-board-69-320.jpg)