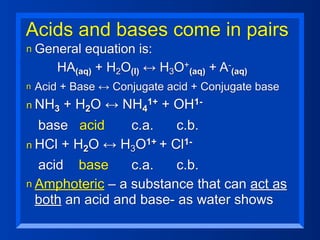
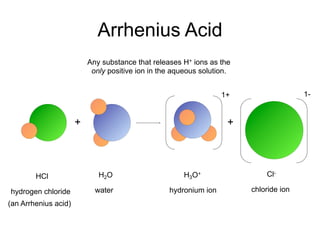
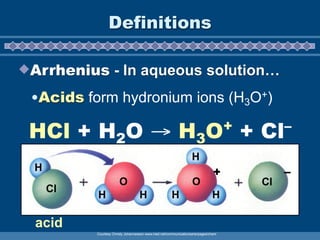
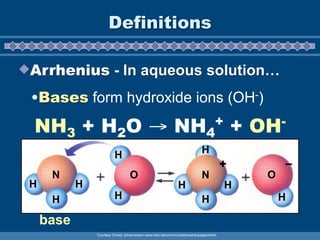
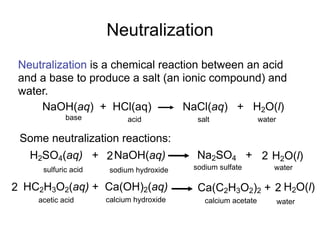
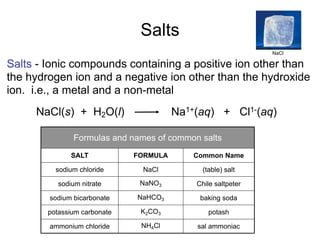
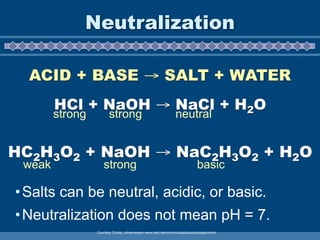
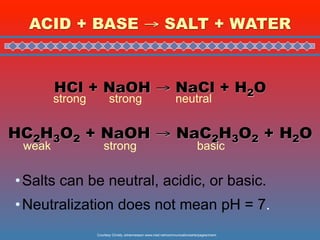
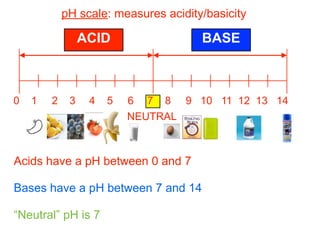
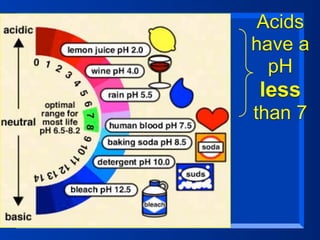
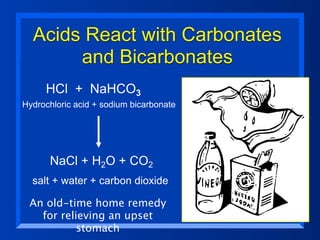
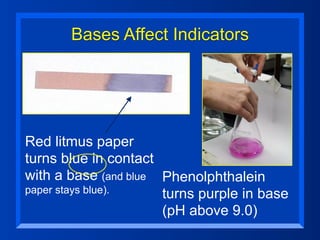
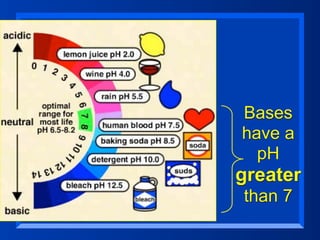
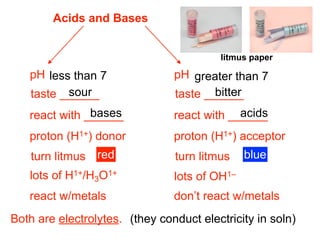
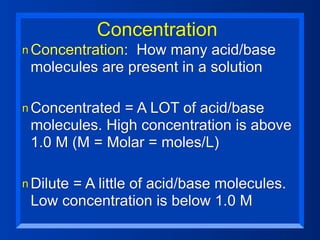
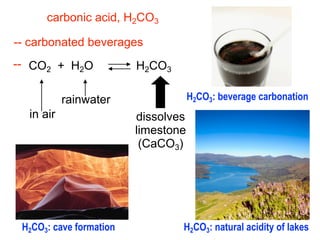
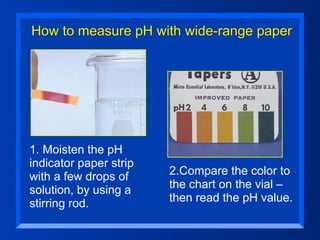
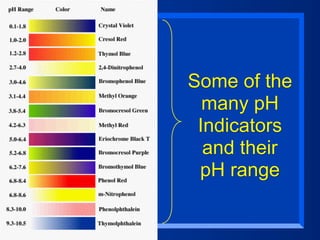
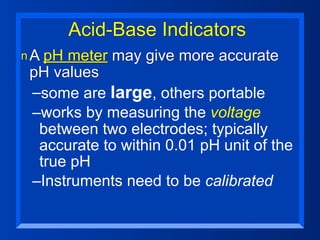
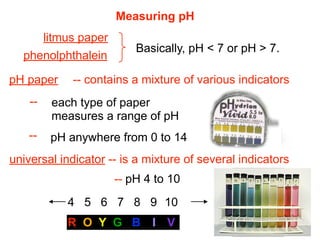
This document discusses the chemistry of acids and bases. It outlines three definitions of acids and bases: Arrhenius, Brønsted-Lowry, and Lewis. The Arrhenius definition states that acids produce hydrogen ions in water and bases produce hydroxide ions. The Brønsted-Lowry definition broadened this to include acids as hydrogen ion donors and bases as acceptors. The Lewis definition focuses on electron pair donation and acceptance. Acids react with bases to form salts and water in a neutralization reaction. Common acids include sulfuric acid, nitric acid, hydrochloric acid, and acetic acid.