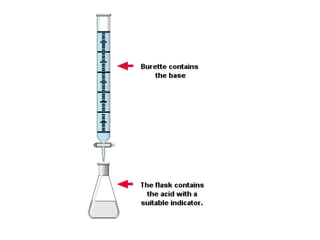
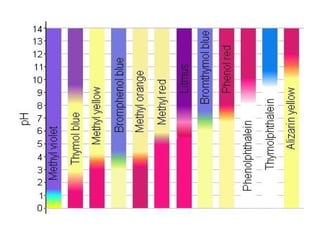
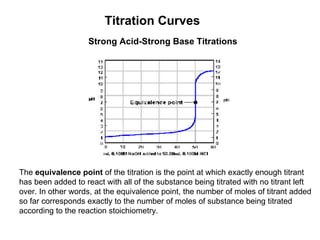
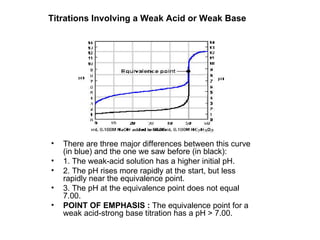
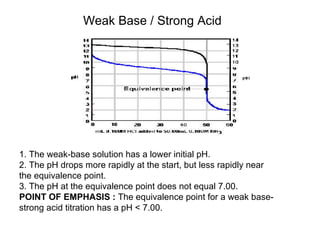
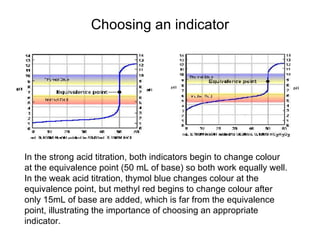
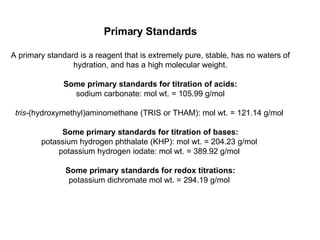
Titration is a technique used to determine the concentration of an unknown substance by reacting it with a known quantity of a titrant. The equivalence point occurs when stoichiometric amounts of the reactants have reacted. An indicator is used to identify the endpoint, which may differ from the equivalence point. The pH at the equivalence point provides information about whether a strong acid/base or weak acid/base reaction took place.