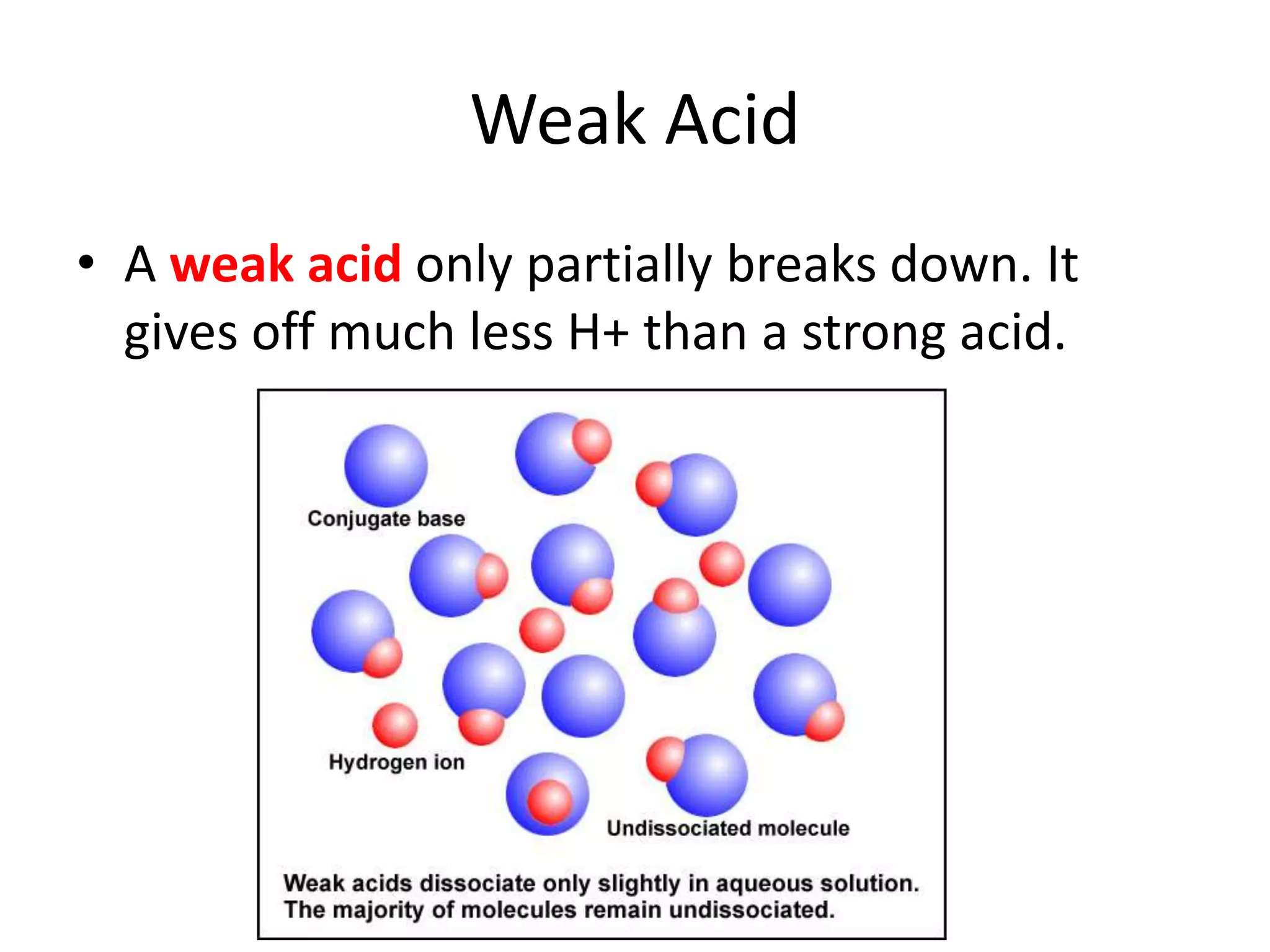
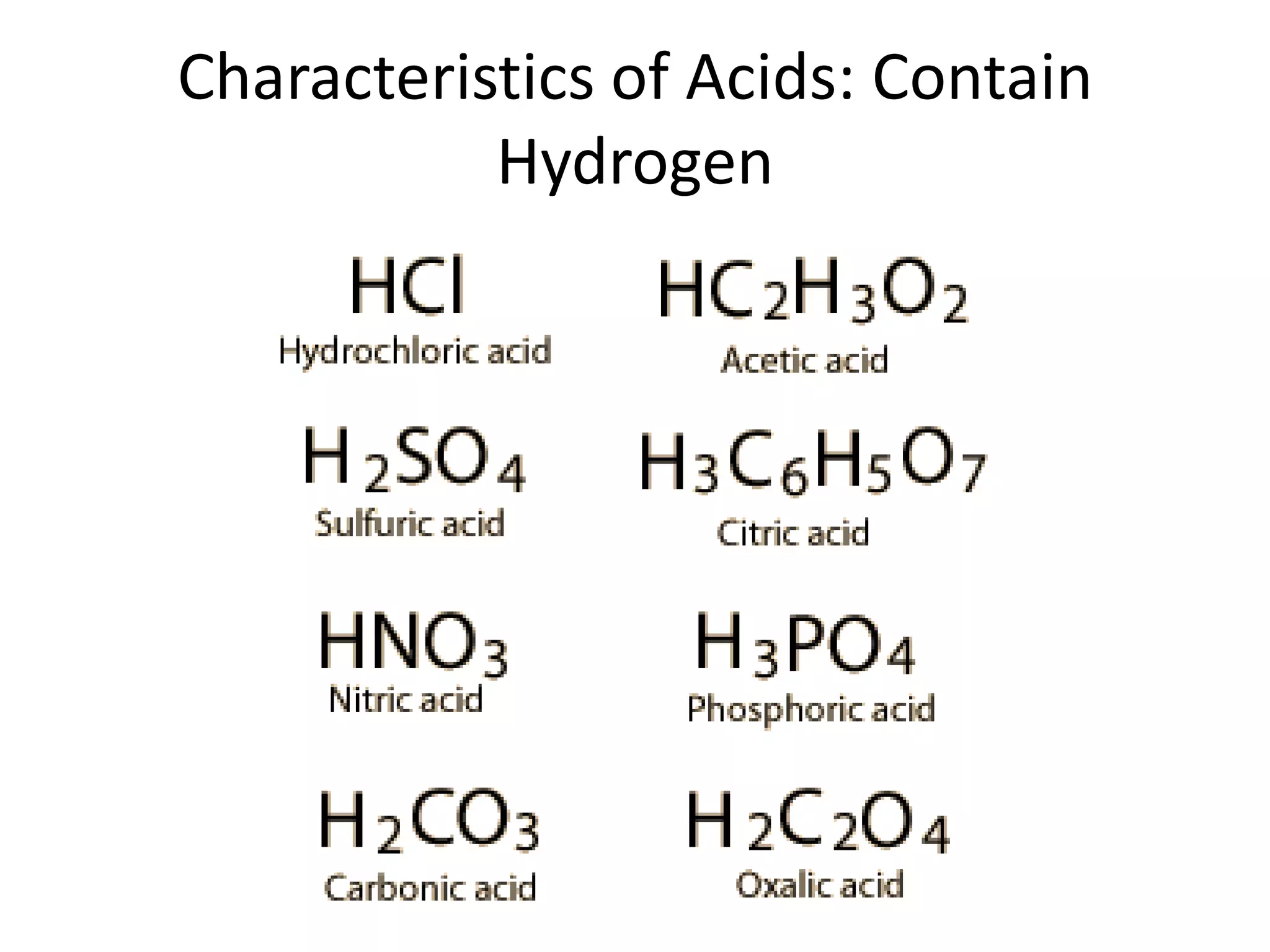
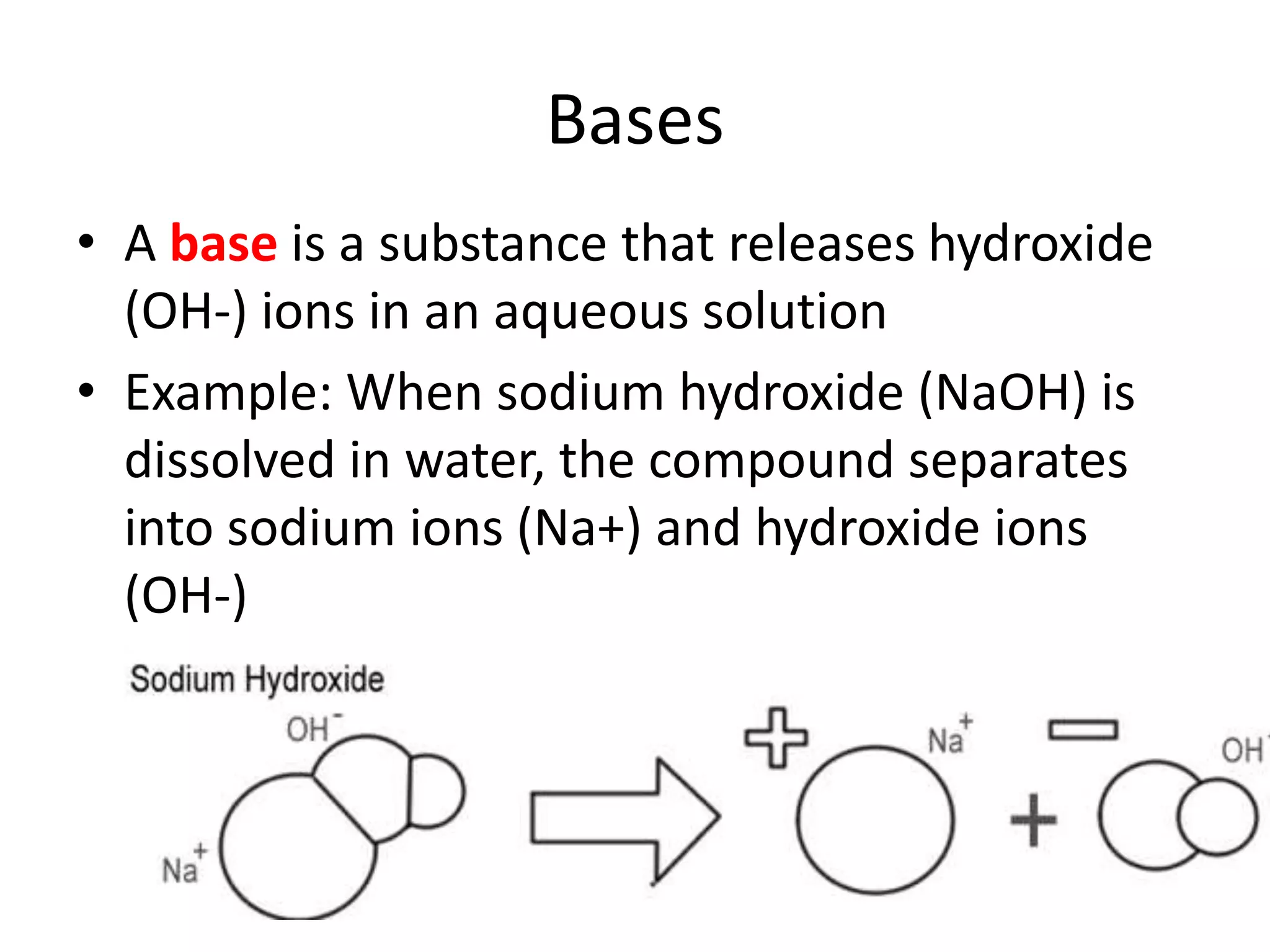
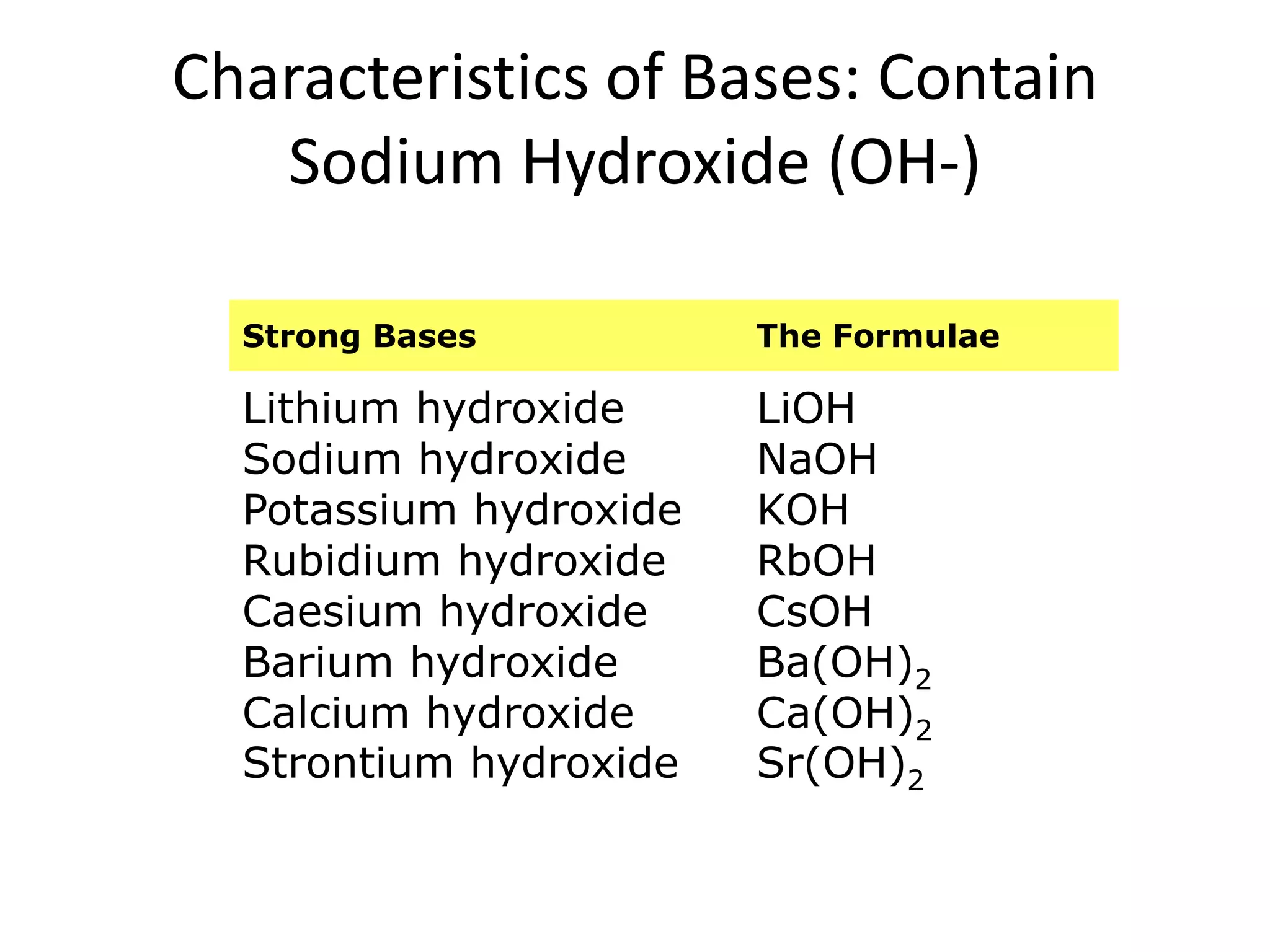
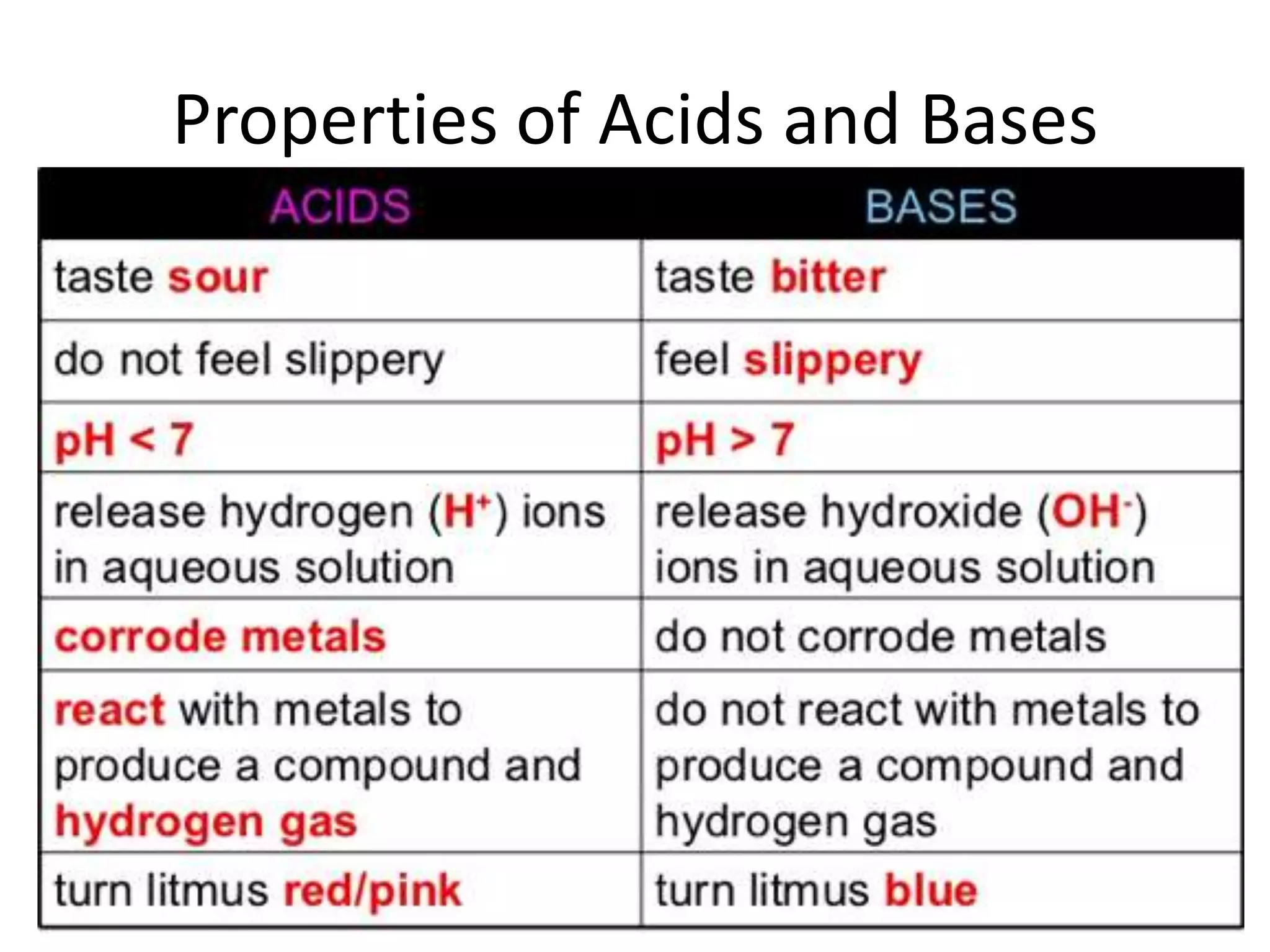
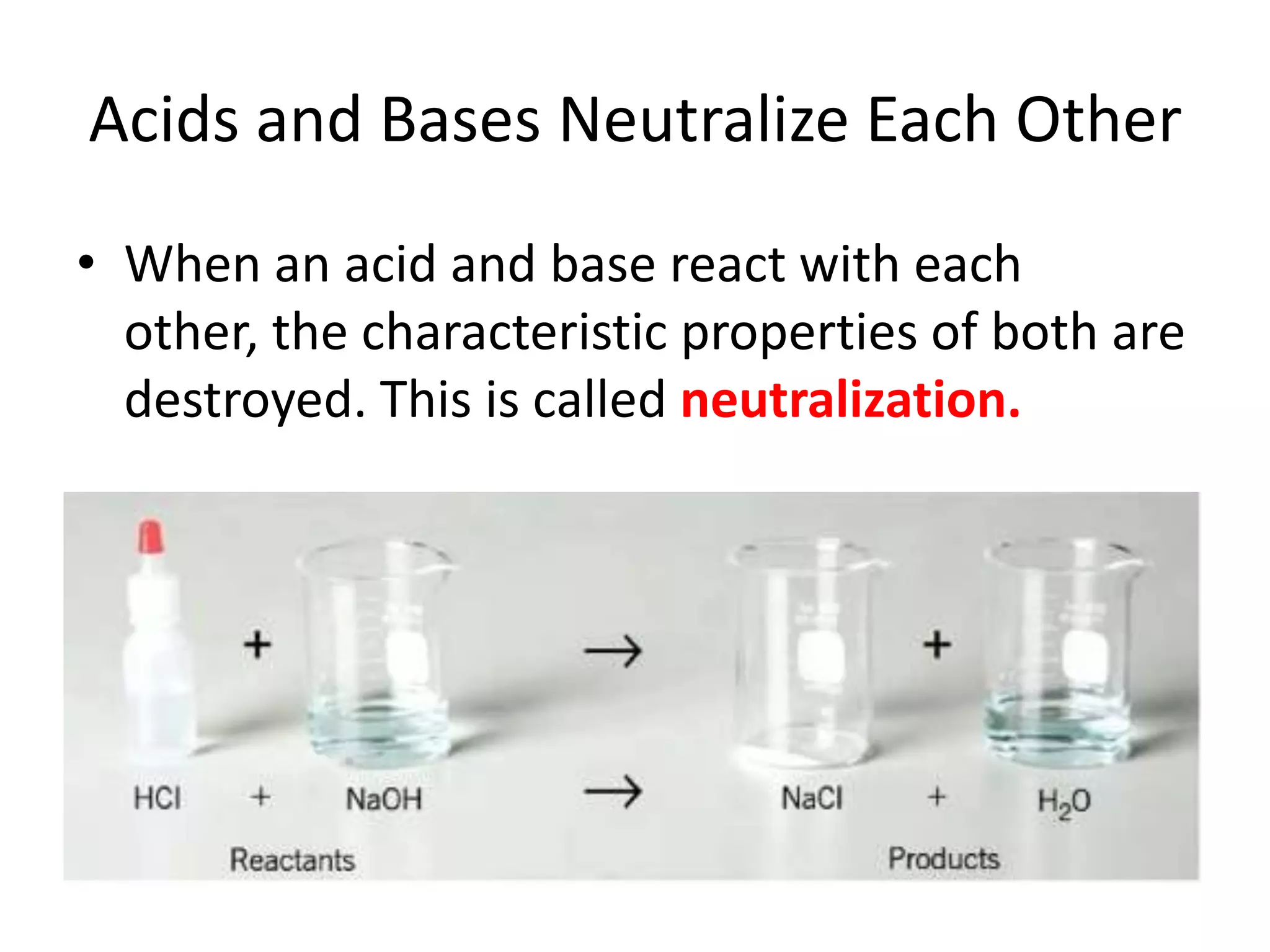
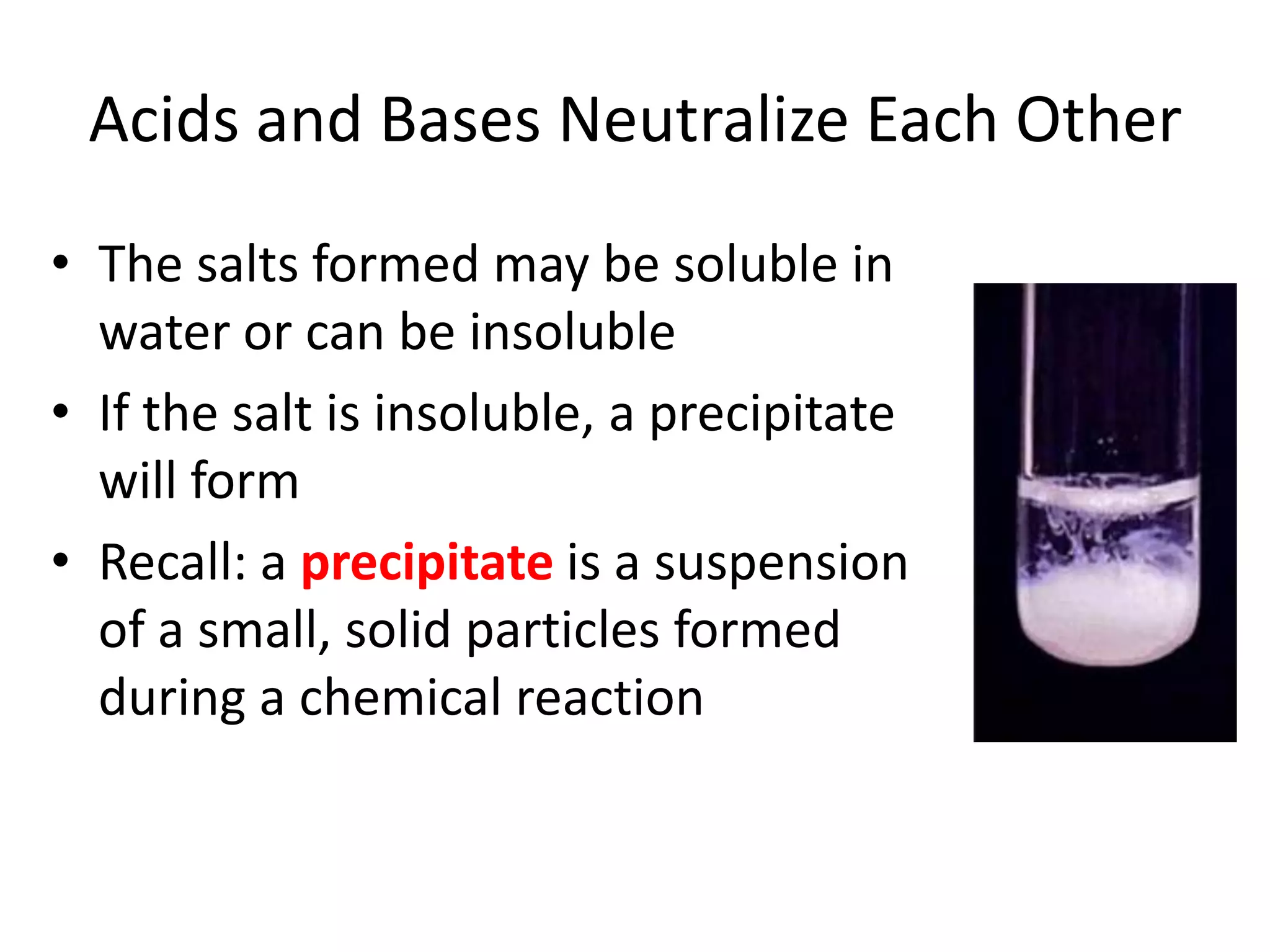
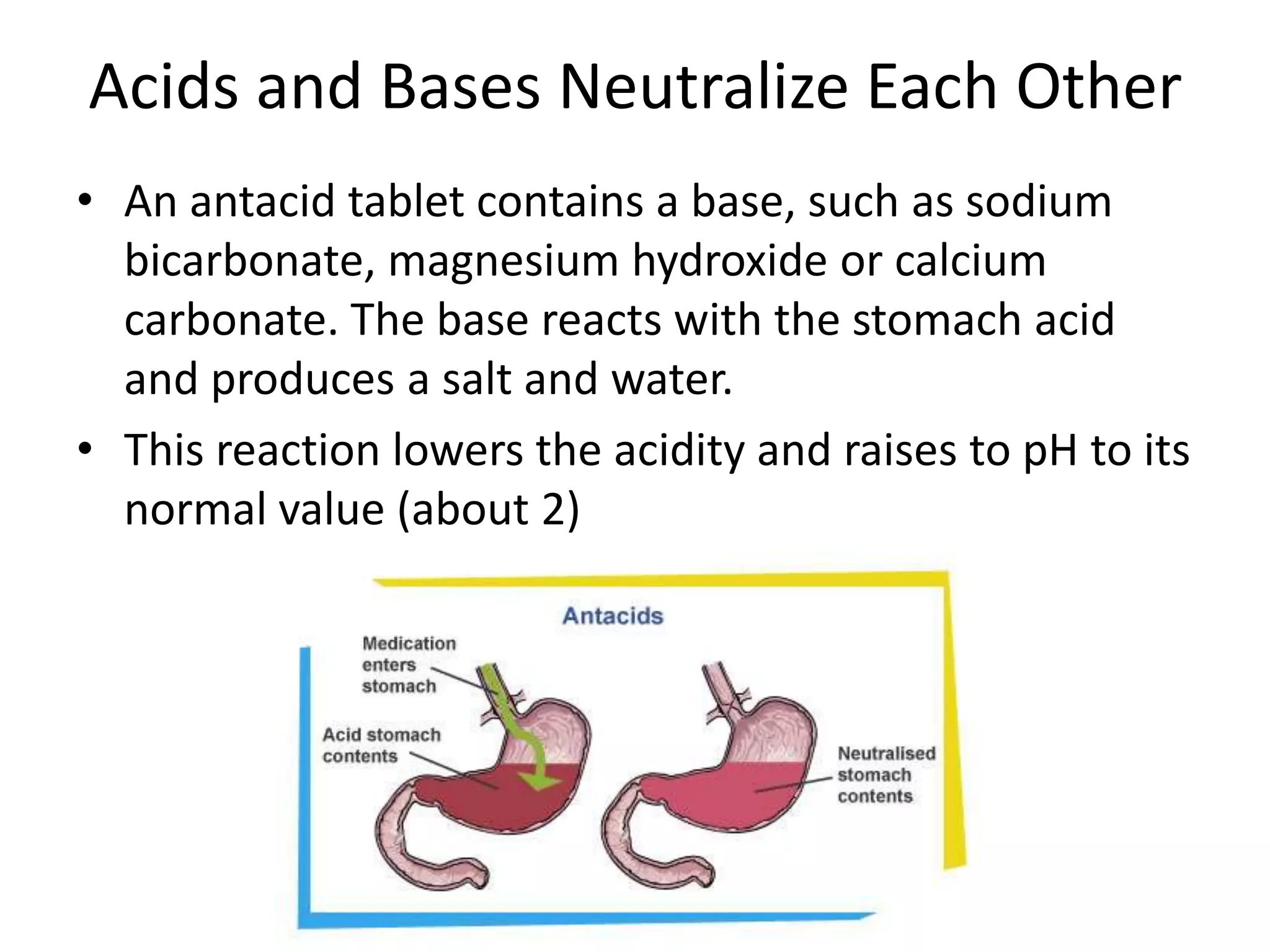
1) Acids release H+ ions in water and bases release OH- ions. Strong acids and bases dissociate completely while weak acids and bases only partially dissociate.
2) Acids taste sour, turn litmus red, and react with metals and carbonates. Bases taste bitter and feel slippery.
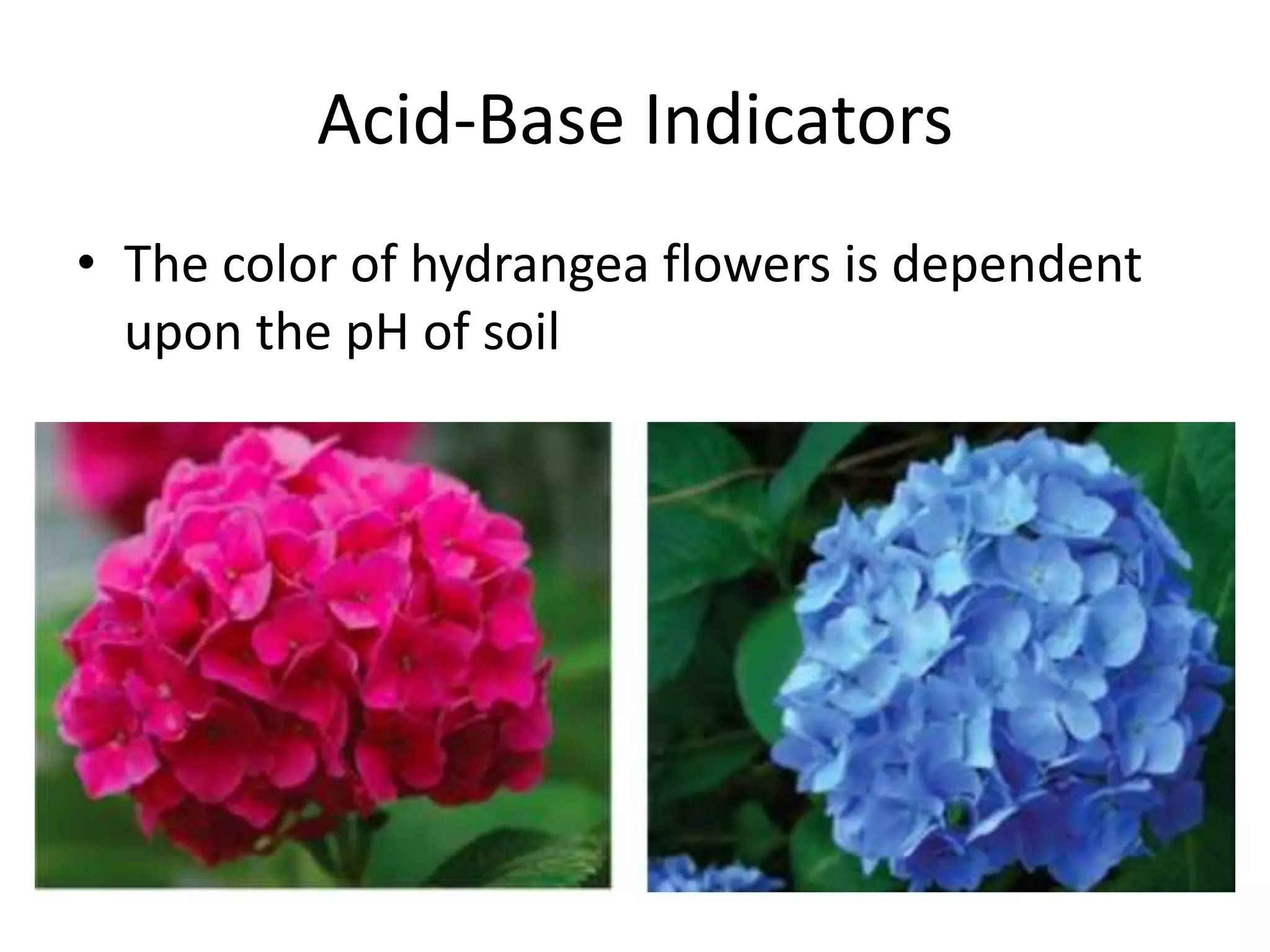
3) The pH scale measures acidity and alkalinity, with values below 7 being acidic and above 7 being basic. Acid-base indicators change color at specific pH values.