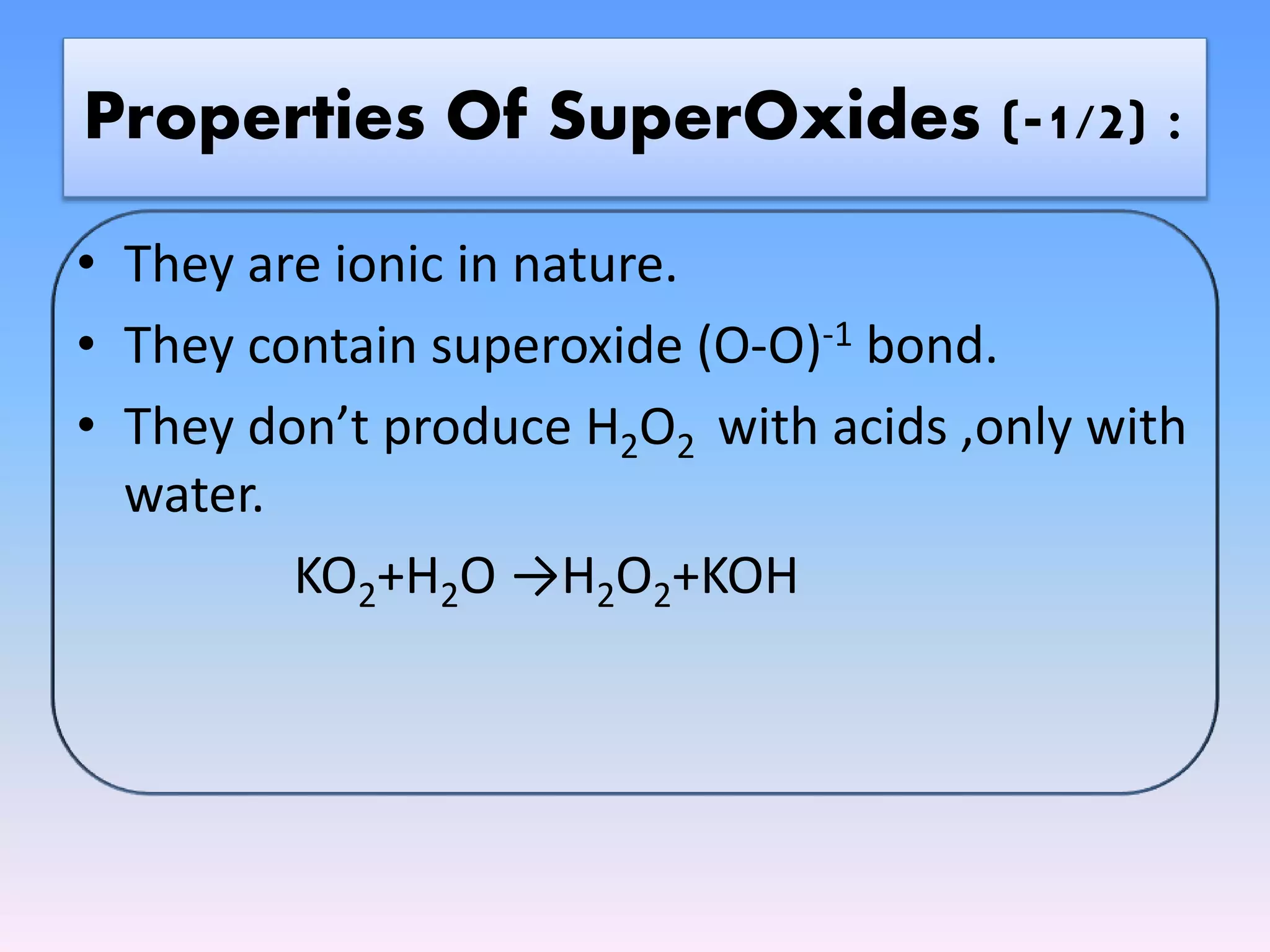
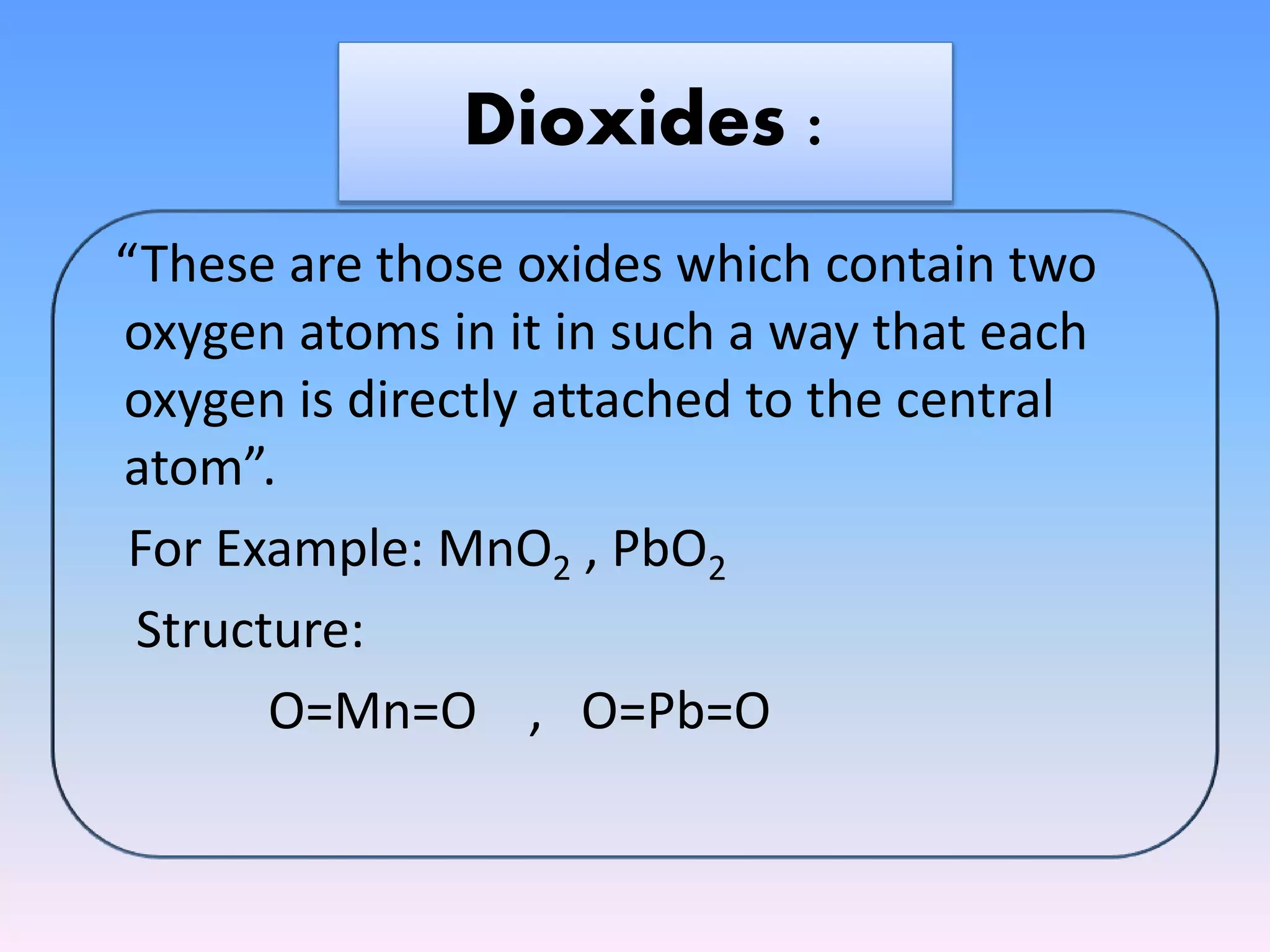
The document outlines the classification of oxides into four main categories: acidic, basic, amphoteric, and neutral oxides, based on their chemical properties and reactions with water. It further details the properties, preparation, and specific examples of each type, including normal oxides, peroxides, superoxides, dioxides, suboxides, and compound oxides. The text discusses the ionic or covalent nature of these oxides and their behavior when reacting with acids and bases.






![Amphoteric Oxides
Amphoteric oxides are compounds of oxygen
which exhibits both acidic as well as basic
characteristics.
• Acidic characteristics:
AL2O3 + 6HCl → AlCl 3+ 3H2O
• Basic characteristics:
Al2O3 +2OH– + 3H2O → 2[Al (OH)4]–](https://image.slidesharecdn.com/oxidesandtheirclassification-200911033620/75/Oxides-and-their-classification-7-2048.jpg)