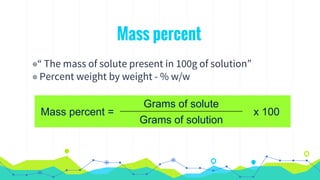
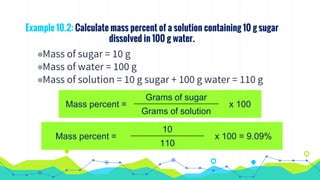
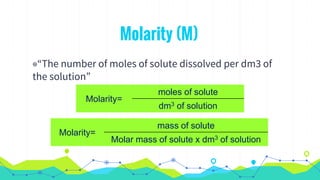
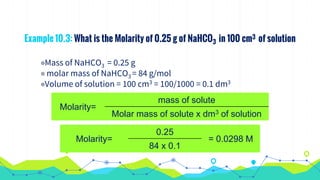
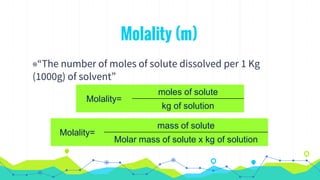
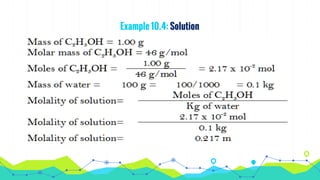
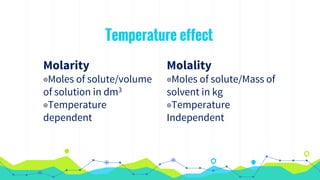
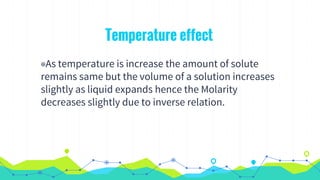
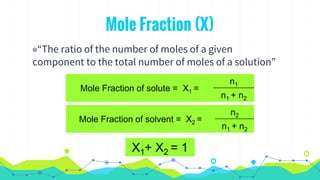
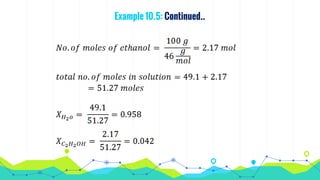
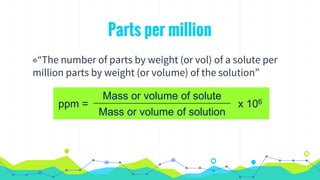
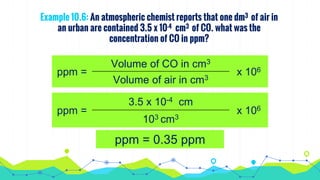
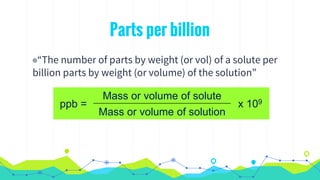
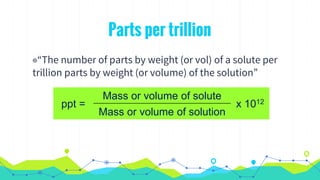
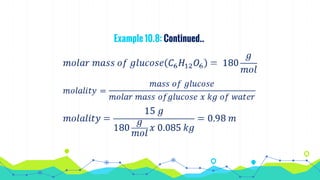
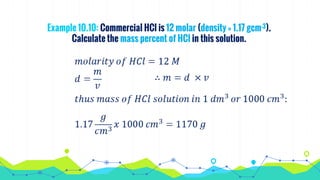
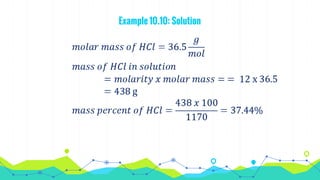
This document discusses various units used to express the concentration of solutions, including mass percent, molarity, molality, mole fraction, and parts per million/billion/trillion. It provides definitions and examples of calculating each type of concentration unit. Various concentration units can be interconverted, as demonstrated through examples solving for molarity, mass percent, and mole fraction in different solutions. Dilution of solutions is also discussed, with an example of calculating molarity after mixing a more concentrated solution with water.