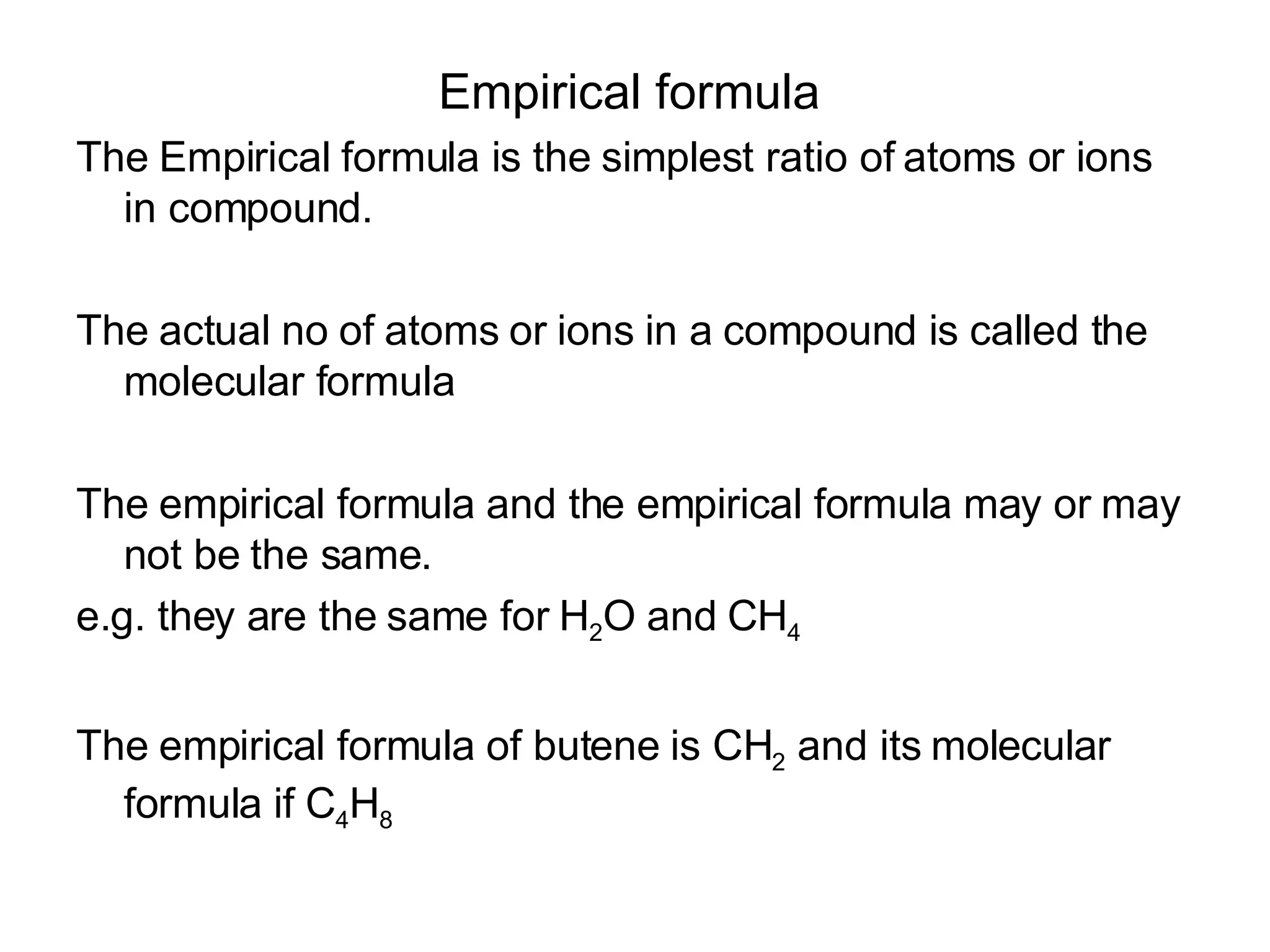
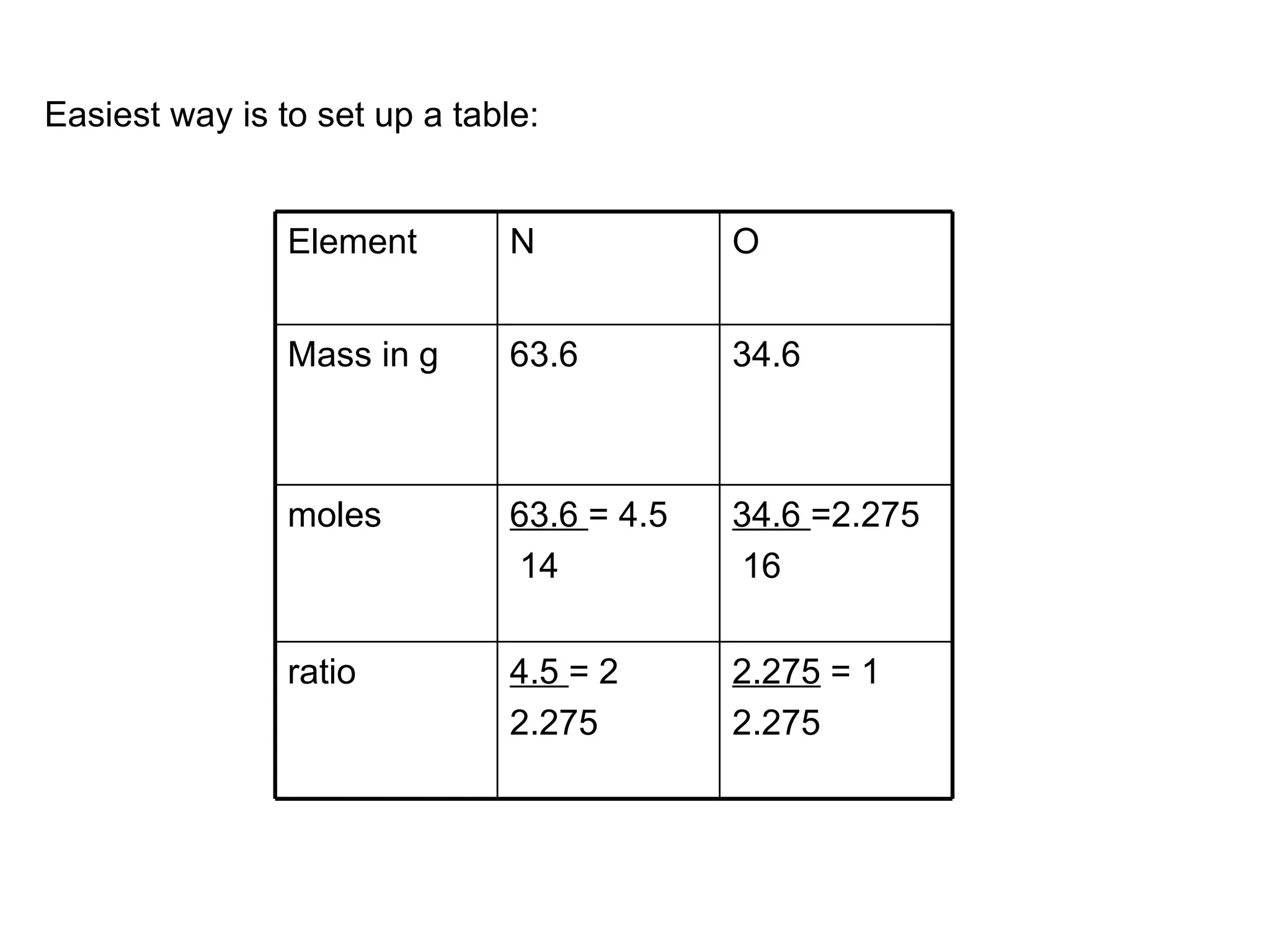
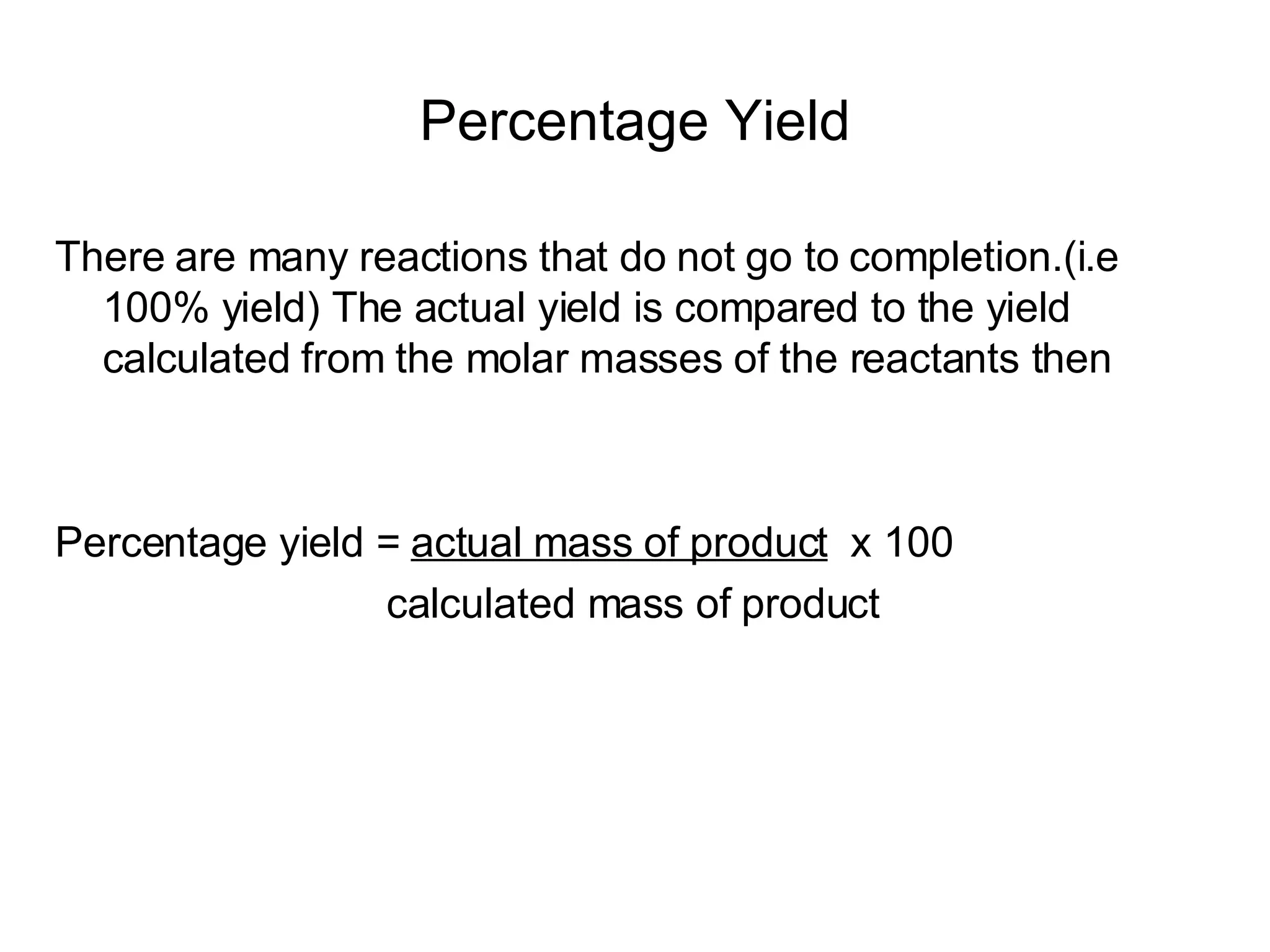
The document discusses empirical formulas, stoichiometry, percentage yield calculations, and identifying limiting reagents. It provides examples of calculating empirical formulas from elemental composition percentages. It also gives examples of finding the limiting reagent, calculating theoretical yield, and determining percentage yield in chemical reactions.