1) Organic chemistry is the study of carbon compounds and their properties. It is a separate discipline due to the vast number and variety of organic compounds, many of which are essential to life.
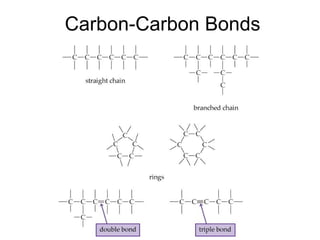
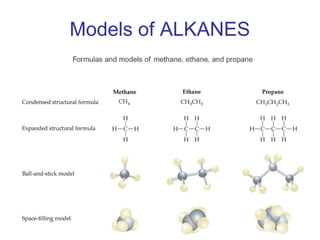
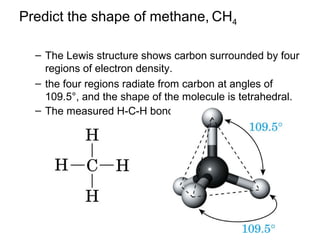
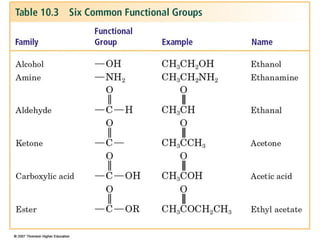
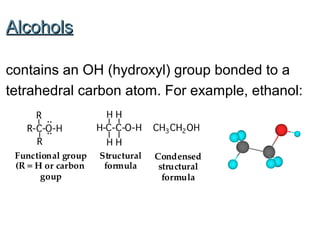
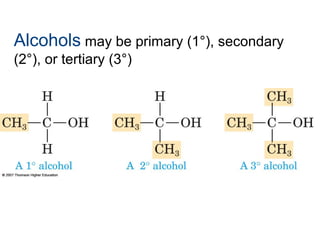
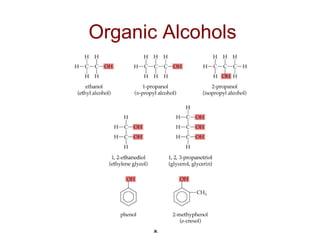
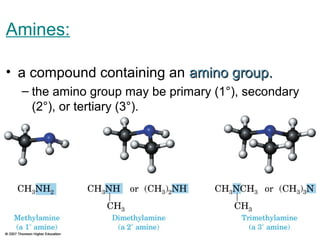
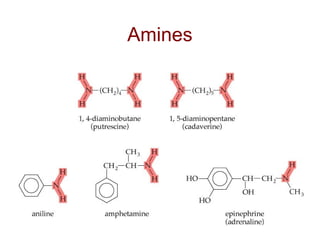
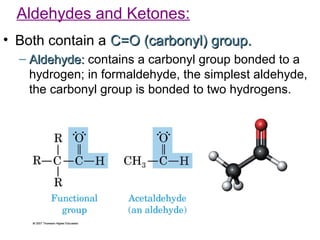
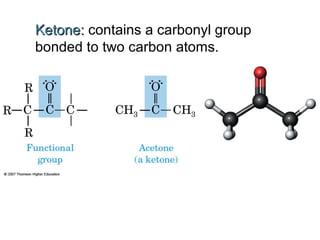
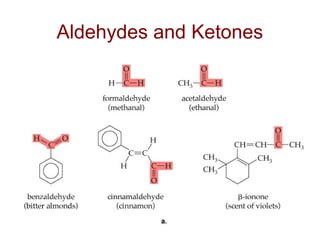
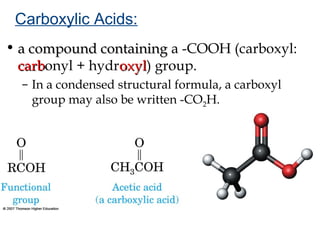
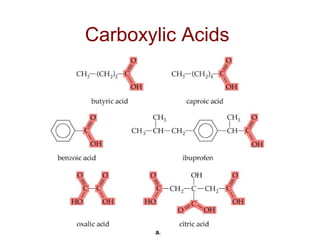
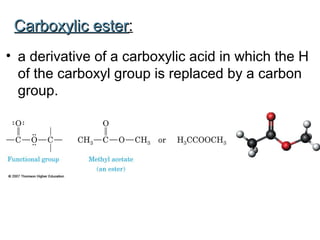
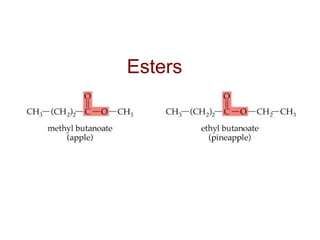
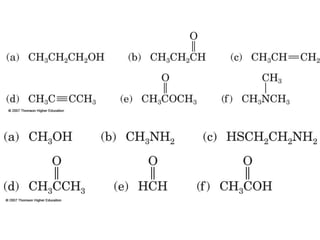
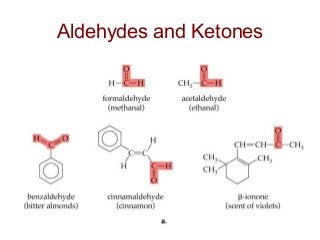
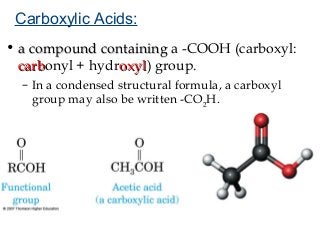
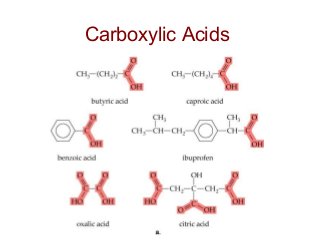
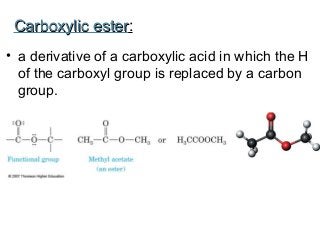
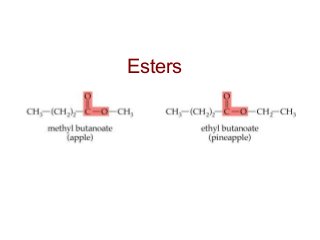
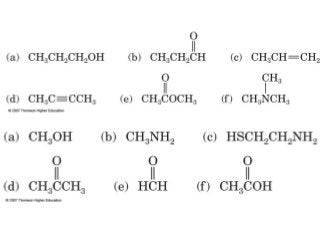
2) Carbon can form chains and rings by bonding to itself and other elements like hydrogen, oxygen, nitrogen and halogens. Functional groups like alcohols, aldehydes, ketones and carboxylic acids determine the properties and reactivity of organic molecules.
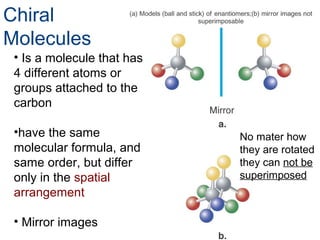
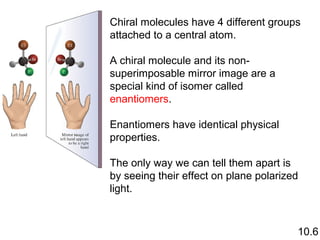
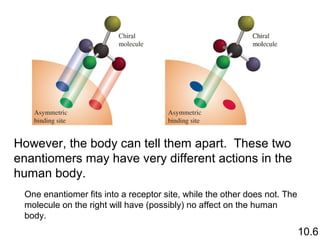
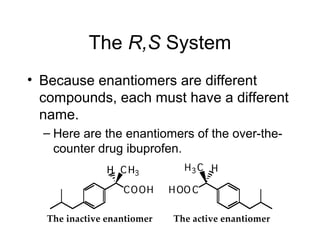
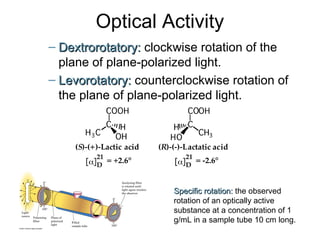
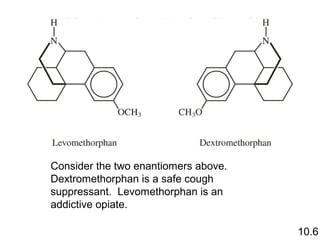
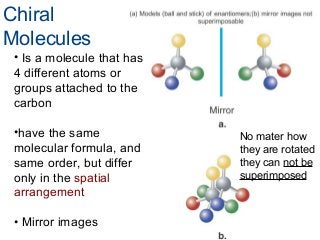
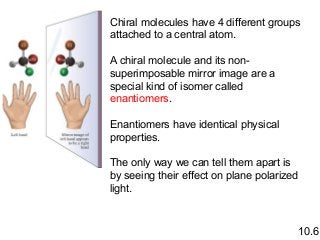
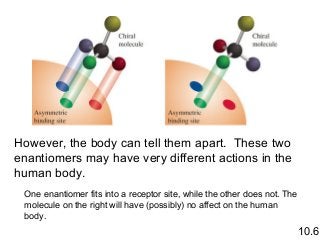
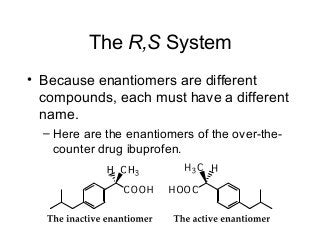
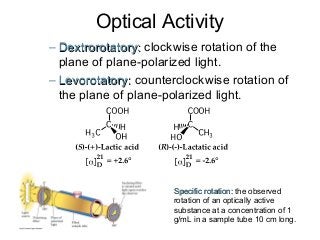
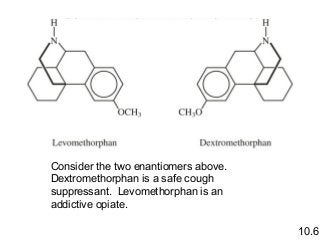
3) Chiral molecules are non-superimposable mirror images called enantiomers that can have different biological effects. The R/S system is used to distinguish these two forms.