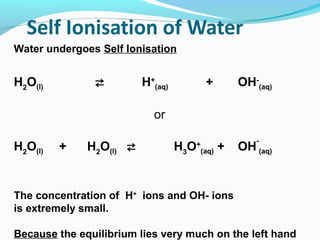
Water undergoes self-ionization in which a small percentage of water molecules dissociate into hydronium (H3O+) and hydroxide (OH-) ions. The concentration of these ions is extremely small and the equilibrium lies very much in the forward direction. The self-ionization of water can be represented by the equilibrium constant Kw, which is equal to the product of the hydronium and hydroxide ion concentrations. Kw is temperature dependent and decreases with increasing temperature. The pH scale was developed to quantify the concentration of hydronium ions in solution and thus indicate whether a solution is acidic, basic, or neutral. pH is defined as the negative logarithm of the hydronium ion concentration
![H2O(l)
⇄
H+(aq) +
Kc
OH-(aq)
=
In the above expression, the value of [H2O] may be taken as having a
constant value because the degree of ionisation is so small.
Kc
=
Kc [H2O] = [H+] [OH-]
Both Kc and [H2O] are constant values so
Kw = Kc [H2O] = [H+] [OH-]](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-2-320.jpg)


![Acid–Base Concentrations in Solutions
concentration (moles/L)
10-1
OH-
H+
10-7
H+
OH-
OH-
H+
10-14
[H+] > [OH-]
[H+] = [OH-]
acidic
solution
neutral
solution
[H+] < [OH-]
basic
solution](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-5-320.jpg)



![At 250C
Kw = 1 x 10-14 mol2/litre2
[H+ ] x [OH- ] = 1 x 10-14 mol2/litre2
This equilibrium constant is very important because it
applies to all aqueous solutions - acids, bases, salts,
and non-electrolytes - not just to pure water.](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-12-320.jpg)
![For H2O(l) ⇄
H+(aq) + OH-(aq)
→
[H+ ] =
[H+ ] x [OH- ] = 1 x 10-14
[OH- ]
= [1 x 10-7 ] x [1 x 10-7 ]
[H+ ] of water is at 250C is 1 x 10-7 mol/litre
Replacing [H+ ] with pH to indicate acidity of solutions
pH 7 replaces [H+ ] of 1 x 10-7 mol/litre
where
pH =
- Log10 [H+ ]](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-13-320.jpg)
![Acid – Base Concentrations and pH
concentration (moles/L)
10-1
pH = 11
pH = 3
OH-
H+
pH = 7
10-7
H+
OH-
OH-
H+
10-14
[H3O+] > [OH-]
acidic
solution
[H3O+] = [OH-]
neutral
solution
[H3O+] < [OH-]
basic
solution](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-15-320.jpg)
![pH describes both [H+ ] and [OH- ]
0
Acidic [H+ ] = 100
[OH- ] =10-14
pH
Neutral
[H+ ] = 10-7
[OH- ] =10-7
pH
Basic
[H+ ] = 10-14
pH
= 14
= 0
= 7
[OH- ] = 100](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-16-320.jpg)
![More acidic
More basic
pH
NaOH, 0.1 M
Household bleach
Household ammonia
Lime water
Milk of magnesia
Borax
Baking soda
Egg white, seawater
Human blood, tears
Milk
Saliva
Rain
Black coffee
Banana
Tomatoes
Wine
Cola, vinegar
Lemon juice
Gastric juice
14
13
12
11
10
9
8
7
6
5
4
3
2
1
0
[H+]
[OH-]
pOH
1 x 10-14
1 x 10-13
1 x 10-12
1 x 10-11
1 x 10-10
1 x 10-9
1 x 10-8
1 x 10-7
1 x 10-6
1 x 10-5
1 x 10-4
1 x 10-3
1 x 10-2
1 x 10-1
1 x 100
1 x 10-0
1 x 10-1
1 x 10-2
1 x 10-3
1 x 10-4
1 x 10-5
1 x 10-6
1 x 10-7
1 x 10-8
1 x 10-9
1 x 10-10
1 x 10-11
1 x 10-12
1 x 10-13
1 x 10-14
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-19-320.jpg)
![Calculations and practice
• You will need to memorize the following:
[H+] = 10–pH
[OH–] = 10–pOH
pH = – log10[H+]
pOH = – log10[OH–]
pH + pOH = 14](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-20-320.jpg)
![pH Calculations
pH
pH = -log10[H+]
[H+]
[H+] = 10-pH
pH + pOH = 14
pOH
[H+] [OH-] = 1 x10-14
pOH = -log10[OH-]
[OH-] = 10-pOH
[OH-]](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-21-320.jpg)
![pH Exercises
a) pH of 0.02M HCl
pH = – log10 [H+]
= – log10 [0.020]
= 1.6989
= 1.70
b) pH of 0.0050M NaOH
pOH = – log10 [OH–]
= – log10 [0.0050]
= 2.3
pH
= 14 – pOH
= 14 – 2.3
=11.7
c) pH of solution where [H +]
is 7.2x10-8M
pH
= – log10 [H+]
= – log10 [7.2x10-8]
= 7.14
(slightly basic)](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-23-320.jpg)
![monoprotic
e.g. HCl, HNO3
HA(aq)
0.3 M
H1+(aq) + A1-(aq)
0.3 M
0.3 M
pH = ?
pH = - log10 [H+]
pH = - log10[0.3M]
pH =
diprotic
e.g. H2SO4
H2A(aq)
0.3 M
2 H1+(aq) + A2-(aq)
0.6 M
0.3 M
0.48
pH = - log10[H+]
pH = - log10[0.6M]
pH =
0.78](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-24-320.jpg)
![A sample of orange juice has a hydrogen-ion
concentration of 2.9 x 10-4M. What is the pH?
pH = -log10 [H+ ]
pH = -log10 (2.9x10-4 )
pH = 3.54](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-25-320.jpg)
![Given: pH = 4.6
determine the [hydrogen ion]
pH = - log10 [H+]
choose proper equation
4.6 = - log10 [H+]
substitute pH value in equation
- 4.6 =
2nd
log
log10[H+]
multiply both sides by -1
- 4.6 =
antilog [H+]
take antilog of both sides
[H+] = 2.51x10-5 M
10x
antilog
You can check your answer by working backwards.
pH = - log10[H+]
pH = - log10[2.51x10-5 M]
pH = 4.6](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-26-320.jpg)

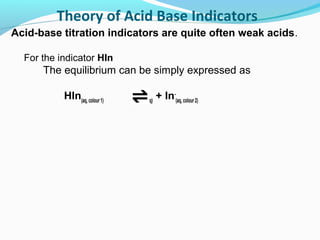
![Theory of Acid Base Indicators
Applying Le Chatelier's equilibrium principle:
Addition of acid
•
favours the formation of more HIn (colour 1)
HIn(aq)
H+(aq) + In-(aq)
because an increase on the right of [H+]
causes a shift to left
increasing [HIn] (colour 1)
to minimise 'enforced' rise in [H+].](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-32-320.jpg)
![Theory of Acid Base Indicators
Applying Le Chatelier's equilibrium principle:
Addition of base
•
favours the formation of more In- (colour 2)
HIn(aq)
H+(aq) + In-(aq)
The increase in [OH-] causes a shift to right
because the reaction
H+(aq) + OH-(aq) ==> H2O(l)
Reducing the [H+] on the right
so more HIn ionises to replace the [H+]
and so increasing In- (colour 2)
to minimise 'enforced' rise in [OH-]](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-33-320.jpg)
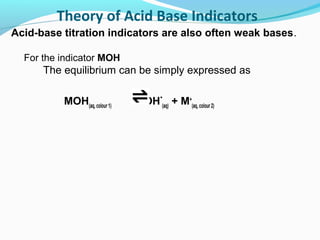
![Theory of Acid Base Indicators
Applying Le Chatelier's equilibrium principle:
Addition of base
•
favours the formation of more MOH (colour 1)
MOH(aq)
M+(aq) + OH-(aq)
because an increase on the right of [OH-]
causes a shift to left
increasing [MOH] (colour 1)
-
to minimise 'enforced' rise in [OH ].](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-35-320.jpg)
![Theory of Acid Base Indicators
Applying Le Chatelier's equilibrium principle:
Addition of acid
•
favours the formation of more M+ (colour 2)
MOH(aq)
M+(aq) + OH-(aq)
The increase in [H+] causes a shift to right
because the reaction
H+(aq) + OH-(aq) ==> H2O(l)
Reducing the [OH-] on the right
so more MOH ionises to replace the [OH-]
and so increasing M+ (colour 2)
+](https://image.slidesharecdn.com/phscale-140309074552-phpapp02/85/Ph-scale-36-320.jpg)