The document discusses factors that affect chemical reaction rates and concepts related to reaction kinetics such as reaction order, rate equations, and rate determining steps. It provides examples of determining the order of reactions from experimental rate data and explains that the slowest step of a reaction, usually the first step, is the rate determining step whose order defines the overall reaction order.


![Decomposition of hydrogen peroxide Rate = gradient = conc example units are mol s -1 time Now we find gradient at various concentrations and plot rate vs concentration [H 2 O 2} ] Time](https://image.slidesharecdn.com/9-reaction-kinetics4722/85/9-Reaction-Kinetics-3-320.jpg)
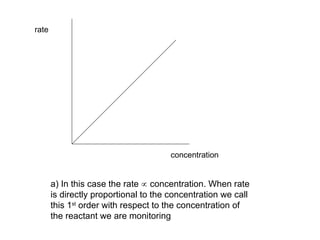


![For a) where the rate is proportional to the conc rate [H 2 O 2 ] or rate = k [H 2 O 2 ] where k is called the rate constant and the above equation is called the rate equation We could write this as rate = k [H 2 O 2 ] 1 but the superscript is always assumed to be 1 if no other number is present. This is the number that is referred to in the expression 1 st order](https://image.slidesharecdn.com/9-reaction-kinetics4722/85/9-Reaction-Kinetics-7-320.jpg)
![Lets suppose we now have a reaction involving 2 reactants A + B products Rate will depend on both [A] and [B] The rate equation is rate = k[A] x [B] y x and y are called the order with respect to A and B X and y may be 0,1,2 (very rarely 3)](https://image.slidesharecdn.com/9-reaction-kinetics4722/85/9-Reaction-Kinetics-8-320.jpg)
![If the order is 0 the the rate does not depend on the concentration of that reactant e.g for the reaction between A and B previously. If order w.r.t A = 1 and order w.r.t B = 0 The rate equation will be Rate =k [A] 1 [B] 0 any number to the power of 0 = 1 Rate equation becomes Rate = k [A]](https://image.slidesharecdn.com/9-reaction-kinetics4722/85/9-Reaction-Kinetics-9-320.jpg)
![The overall order of a reaction is the sum of all the superscripts So for the reaction with a rate equation as follows Rate = k[A] 1 ][B] 2 The overall order is 3 It is important to remember that no information about the order or the rate of a reaction can be found from an equation. All the kinetic data must be found by experiment](https://image.slidesharecdn.com/9-reaction-kinetics4722/85/9-Reaction-Kinetics-10-320.jpg)


![Lets look at A first. When B is kept the same and A is doubled the rate doubles. rate [A] 1 i.e. order w.r.t A = 1 When A kept the same and B doubled The rate increases x 4 (i.e 2 2 ) Rate [B] 2 i.e. order w.r.t B = 2 Overall order = 3](https://image.slidesharecdn.com/9-reaction-kinetics4722/85/9-Reaction-Kinetics-13-320.jpg)
![our rate equation then is rate = k [A][B] 2 As k is a constant it will be the same for any of the 3 experiments so we can use any experiment and put in the values of [A] [B] and initial rate to find k. k = rate k = 3.0 x 10 -3 [A][B] 2 (2 x 10 -3 )(3.0 x 10 -3 ) 2 = 1.67 x 10 5 What are the units? mol/L/h = mol -2 L 2 h -1 (mol/L) 3](https://image.slidesharecdn.com/9-reaction-kinetics4722/85/9-Reaction-Kinetics-14-320.jpg)

![In any reaction the slowest step (usually the first step) is the rate determining step (r.d.s) The order of reaction is the number of molecules or atoms taking part in the r.d.s So in the previous example the order w.r.t. A would be 1 and the overall order would also be 1. the rate depends on the first step so [B] has no effect on the rate.](https://image.slidesharecdn.com/9-reaction-kinetics4722/85/9-Reaction-Kinetics-16-320.jpg)
![For reaction with the following rate equation Rate = k[A][B] Order w.r.t A = 1 Order w.r.t B = 1 1 molecule A and 1 molecule B take part in the r.d.s For a reaction with the following rate equation Rate = k[A] 2 B] 0 This would be a 2 step reaction Step 1 A + A X Step 2 X + B products](https://image.slidesharecdn.com/9-reaction-kinetics4722/85/9-Reaction-Kinetics-17-320.jpg)