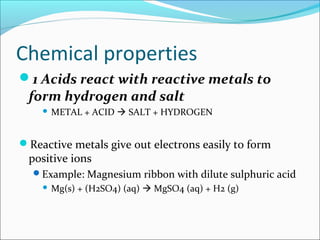
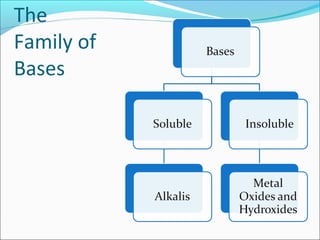
This document defines acids and bases and discusses their properties. It states that acids produce hydrogen ions in water and have a pH less than 7, while bases are metal oxides or hydroxides that react with acids to form salts and water. Common acids include hydrochloric acid and sulfuric acid, while common bases include sodium hydroxide and calcium hydroxide. The document also discusses concentration versus strength, noting that concentration can change but strength depends on the inherent properties of the acid or base.