This document provides information about acids and bases including:
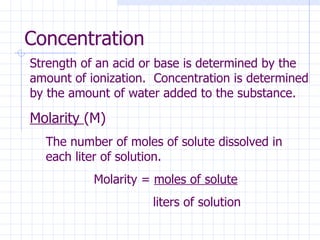
- Acids produce H+ ions in water and bases produce OH- ions. The strength depends on how completely they ionize.
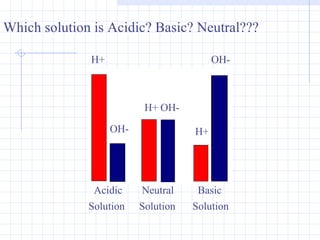
- Examples of acids include vinegar and lemon juice. Examples of bases include soap and ammonia. Neutral substances have equal concentrations of H+ and OH- ions.
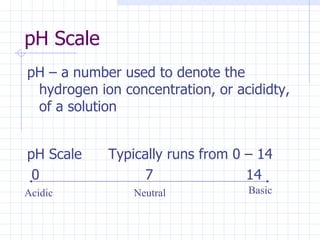
- Acids have a sour taste and low pH while bases have a bitter taste, slippery feel and high pH.
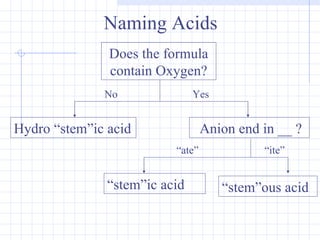
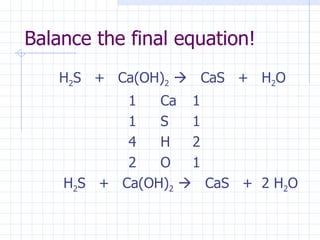
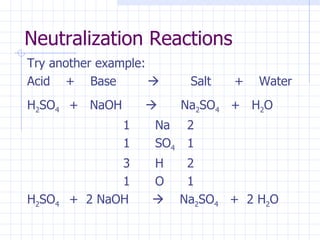
- The names and formulas of acids can be determined based on their ionic parts. Neutralization reactions between acids and bases produce salt and water. pH indicators are used to determine if a solution is acidic or basic.