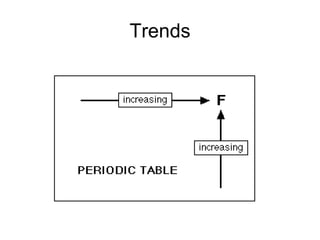
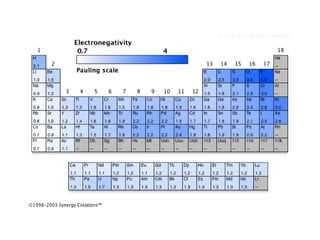
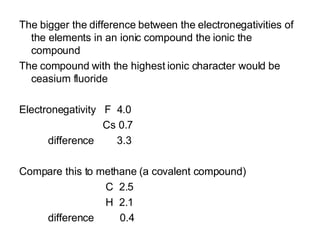
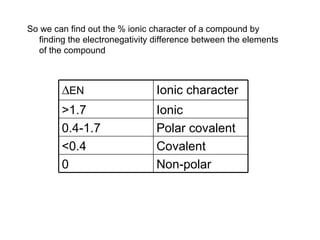
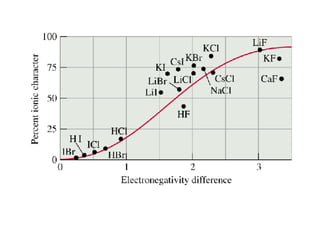
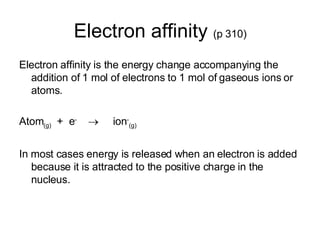
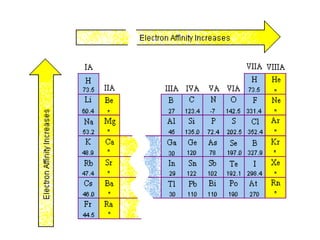
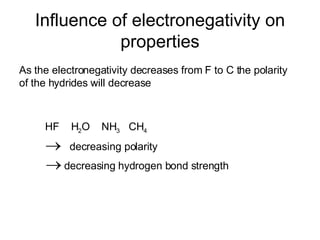
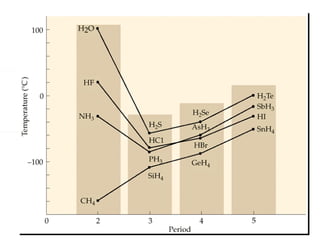
This document discusses electronegativity and electron affinity. It explains that electronegativity is an atom's ability to attract electrons in a covalent bond, and fluorine is the most electronegative while cesium is the least. Electron affinity is the energy change when an electron is added to a gaseous atom or ion. The difference in electronegativity between elements in an ionic compound determines its ionic character. Compounds with a difference greater than 1.7 are considered ionic. Electronegativity also influences molecular properties like polarity and boiling points through hydrogen bonding abilities.