1) Colloidal particles can be stabilized through Brownian motion if particle collisions do not result in sticking.
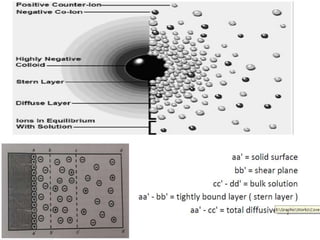
2) The key forces between colloidal particles include van der Waals attraction, electrostatic repulsion, steric repulsion, and solvation forces.
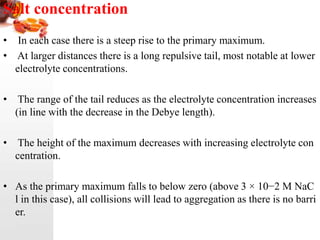
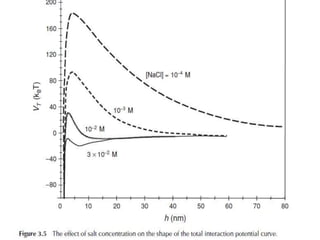
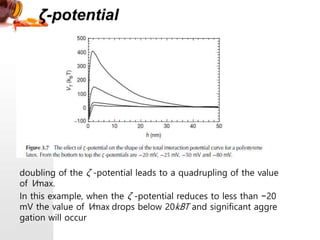
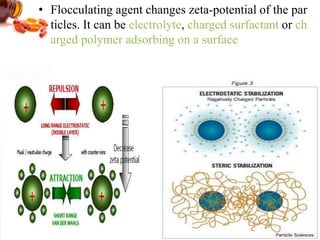
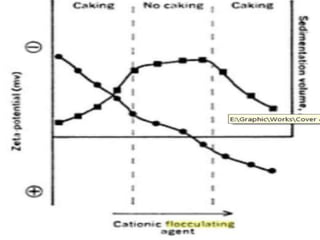
3) The stability of colloids is determined by factors such as salt concentration, particle charge (zeta potential), particle size, and addition of flocculating agents which can reduce zeta potential leading to aggregation.