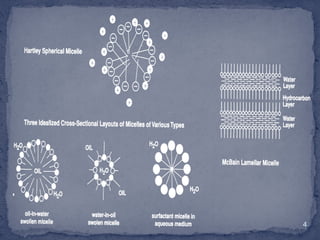
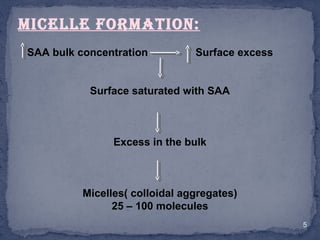
The document discusses micelles, aggregates of surfactant molecules in liquid, focusing on their formation, size, and the critical micelle concentration (CMC). The CMC is crucial for maximizing surfactant performance and is affected by factors such as temperature, molecular structure, and the presence of additives like electrolytes and alcohols. Methods for determining CMC, including conductivity and absorption techniques, are also highlighted.