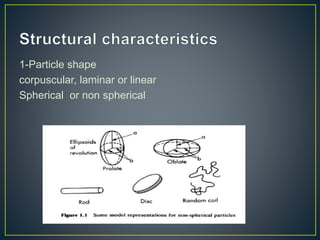
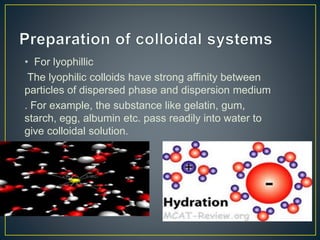
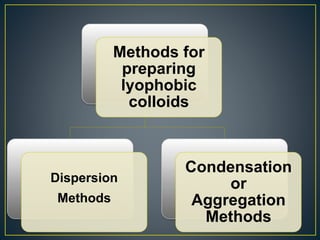
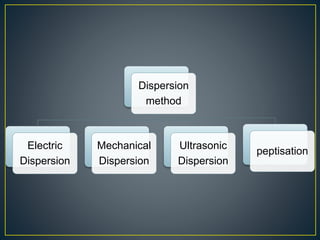
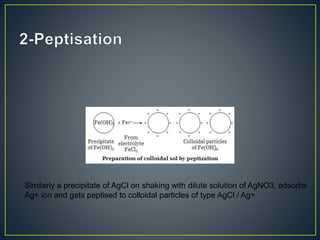
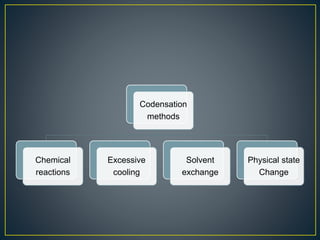
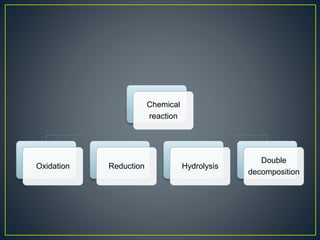
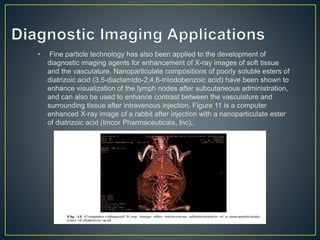
This document provides an overview of colloid science. It defines colloids as mixtures of two phases where one component is dispersed as nanometer- to micrometer-sized particles. The document then classifies and describes the structural characteristics of colloidal systems. It discusses several preparation and purification methods for colloids before explaining the stability of colloidal dispersions. The document concludes by outlining some common applications of colloids in areas like pharmaceuticals, cosmetics, paint, and rubber production.