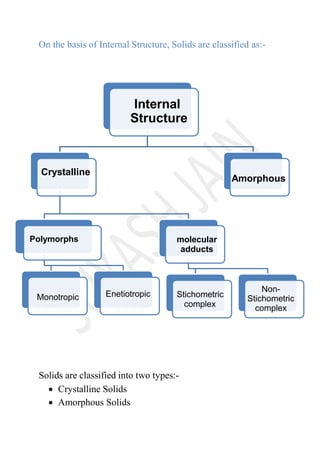
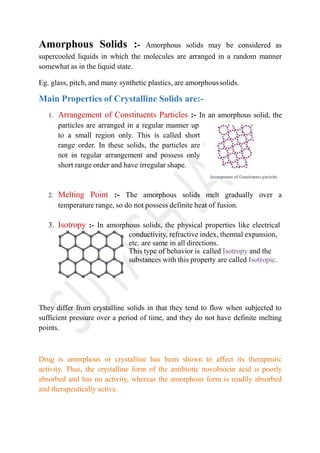
The document discusses the characteristics and classifications of solids, including crystalline and amorphous forms, as well as the concept of polymorphism. Crystalline solids exhibit a structured arrangement of particles with distinct melting points and anisotropic properties, while amorphous solids display random particle arrangements with varying melting points and isotropic properties. The relevance of polymorphism in pharmaceuticals is highlighted, emphasizing how different forms of a substance can affect its solubility and therapeutic activity.