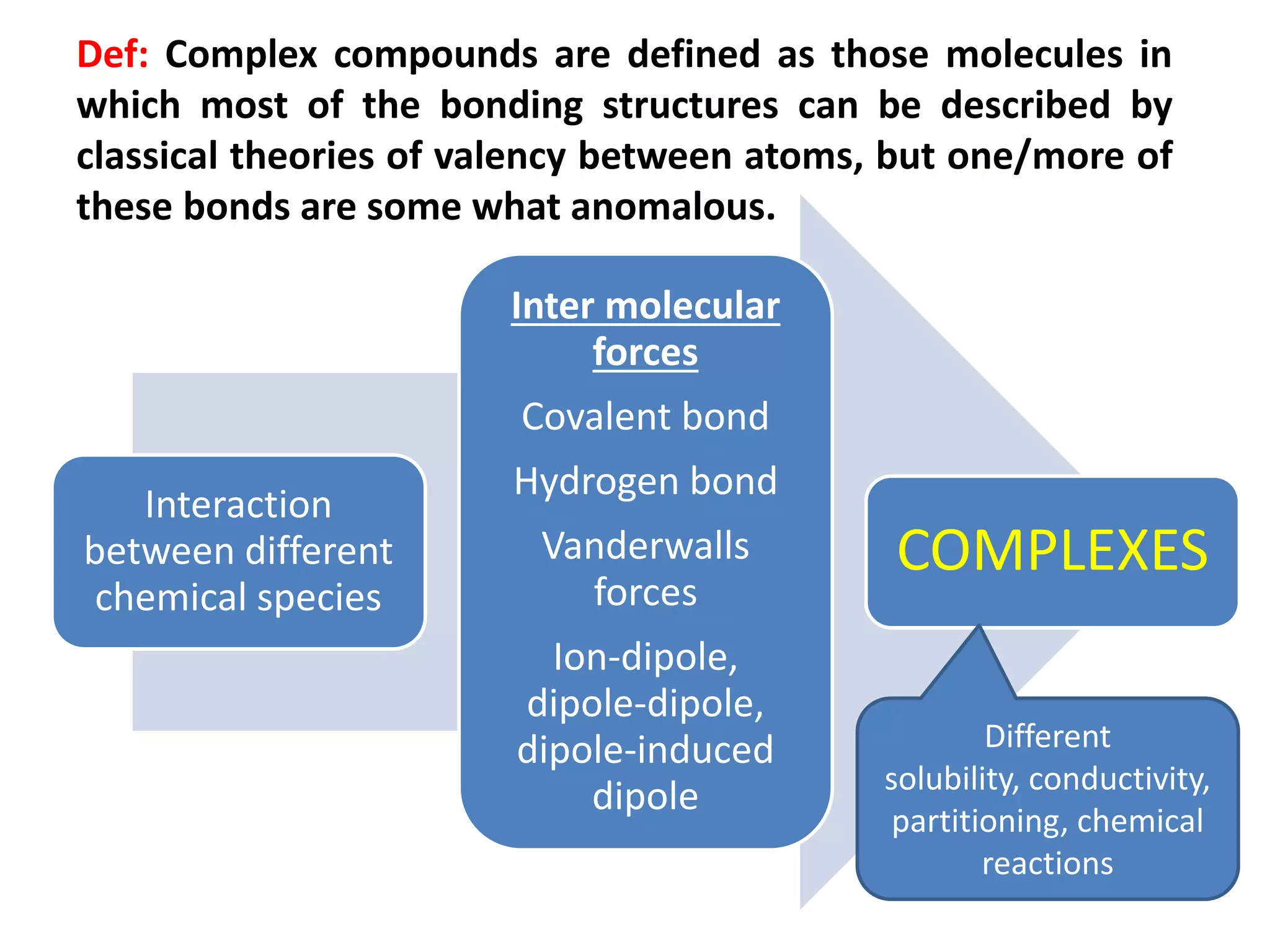
1. Complex compounds are molecules where some bonds cannot be described by classical theories of valency and involve anomalous bonds.
2. Complexes form through interactions like coordination bonds, hydrogen bonds, and van der Waals forces between different chemical species.
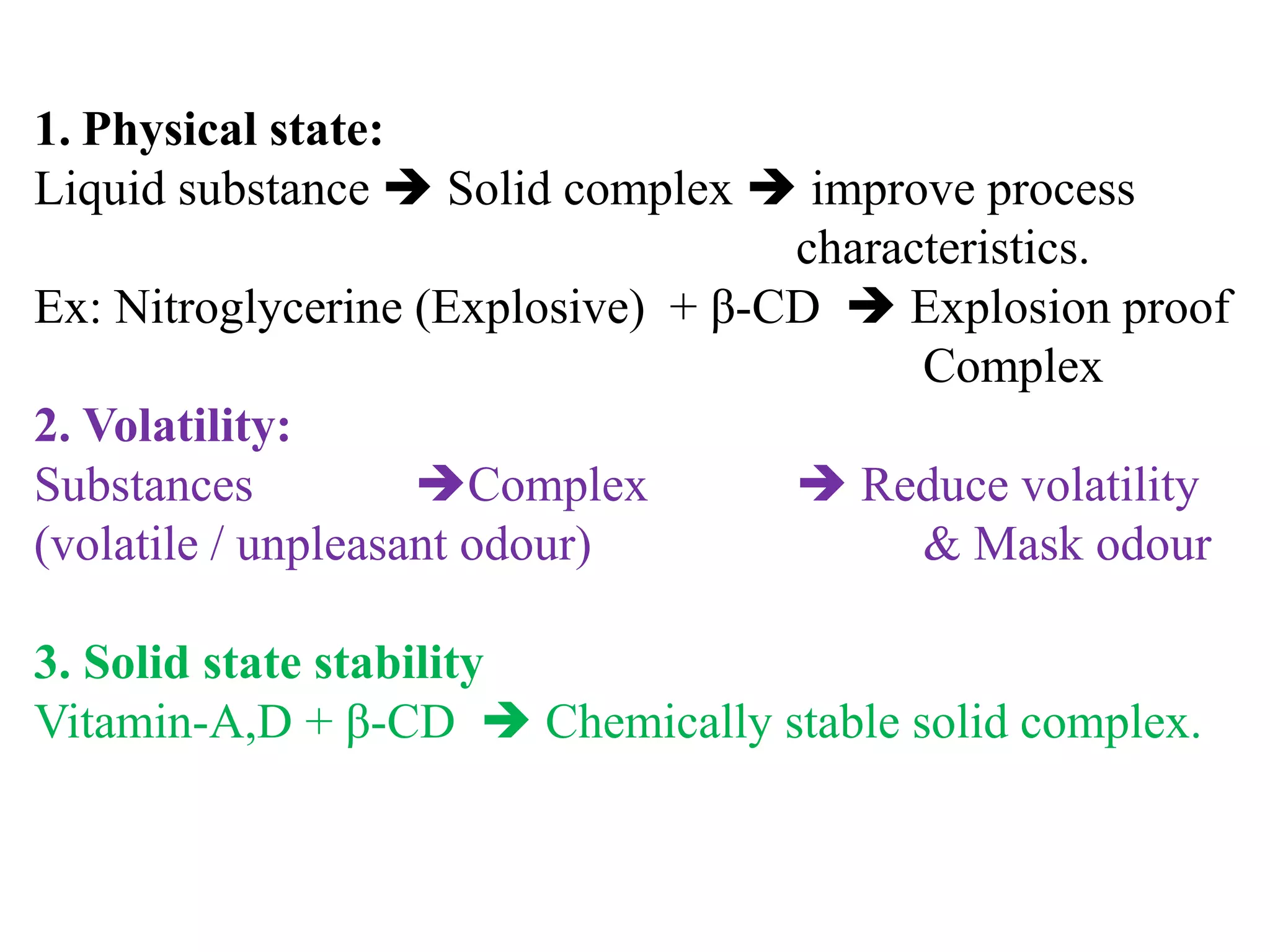
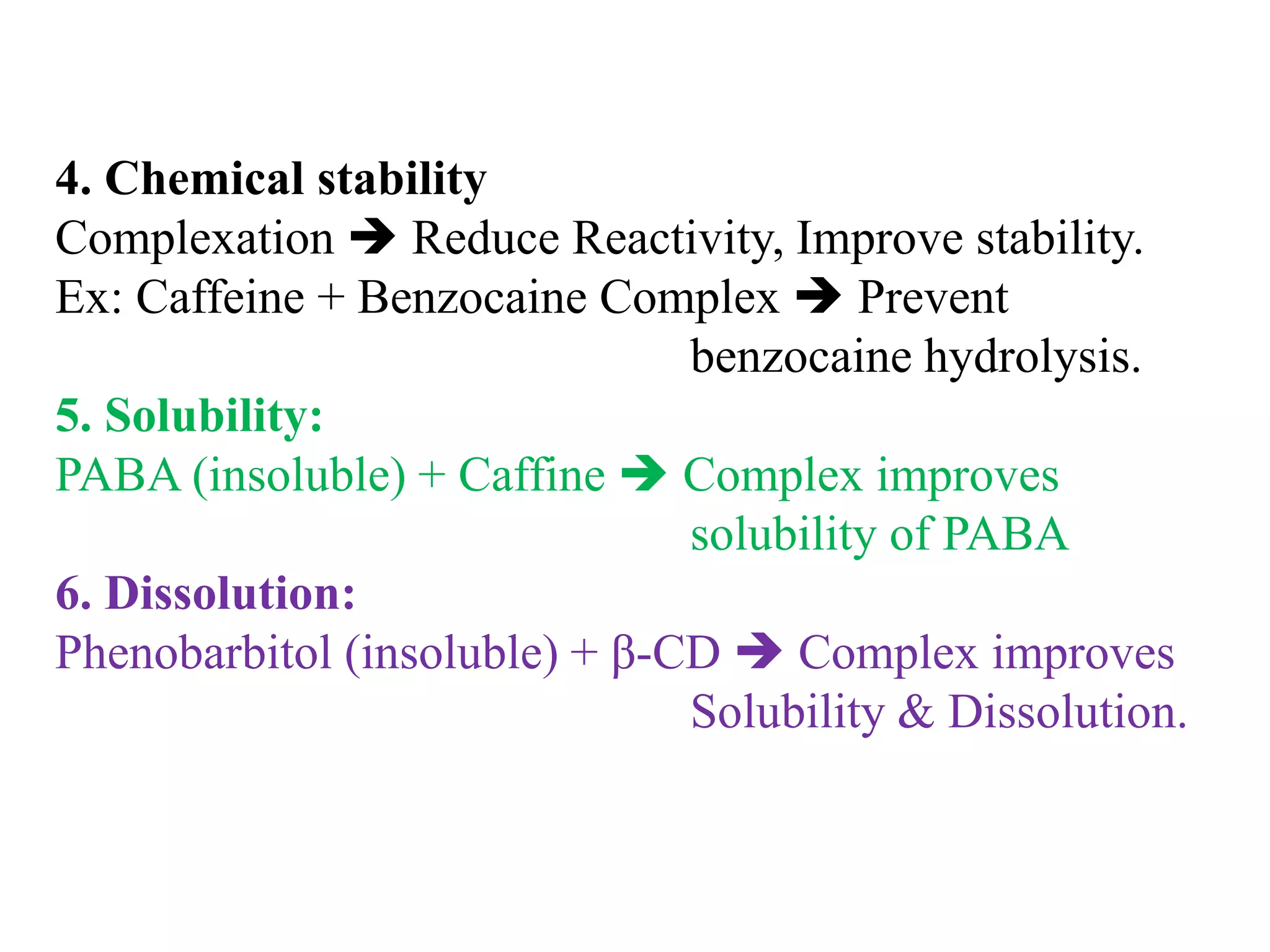
3. Complexation can alter properties like solubility, conductivity, and chemical reactivity and is used in applications like increasing drug solubility, purification of water, drug analysis, and as anticoagulants.


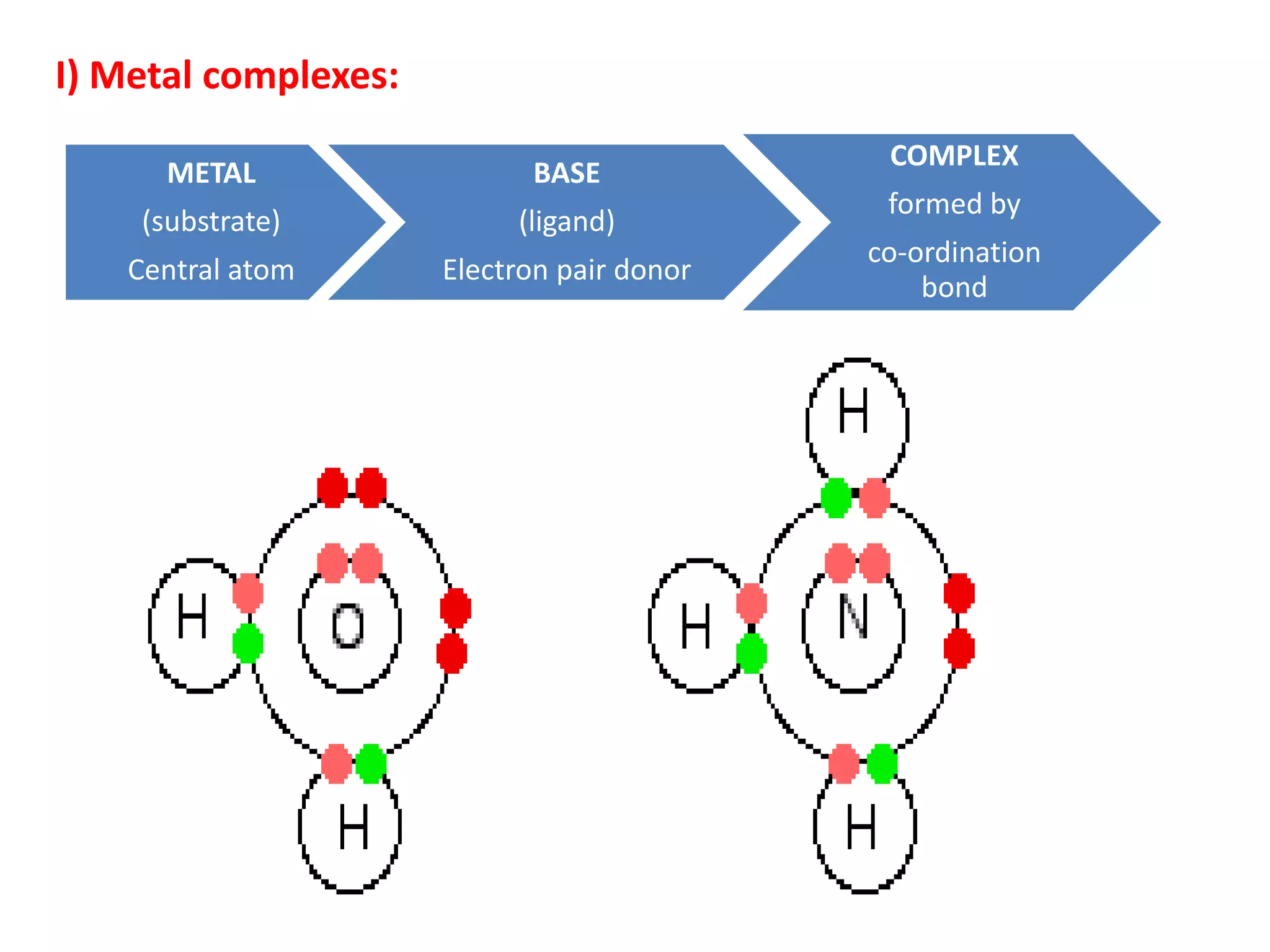
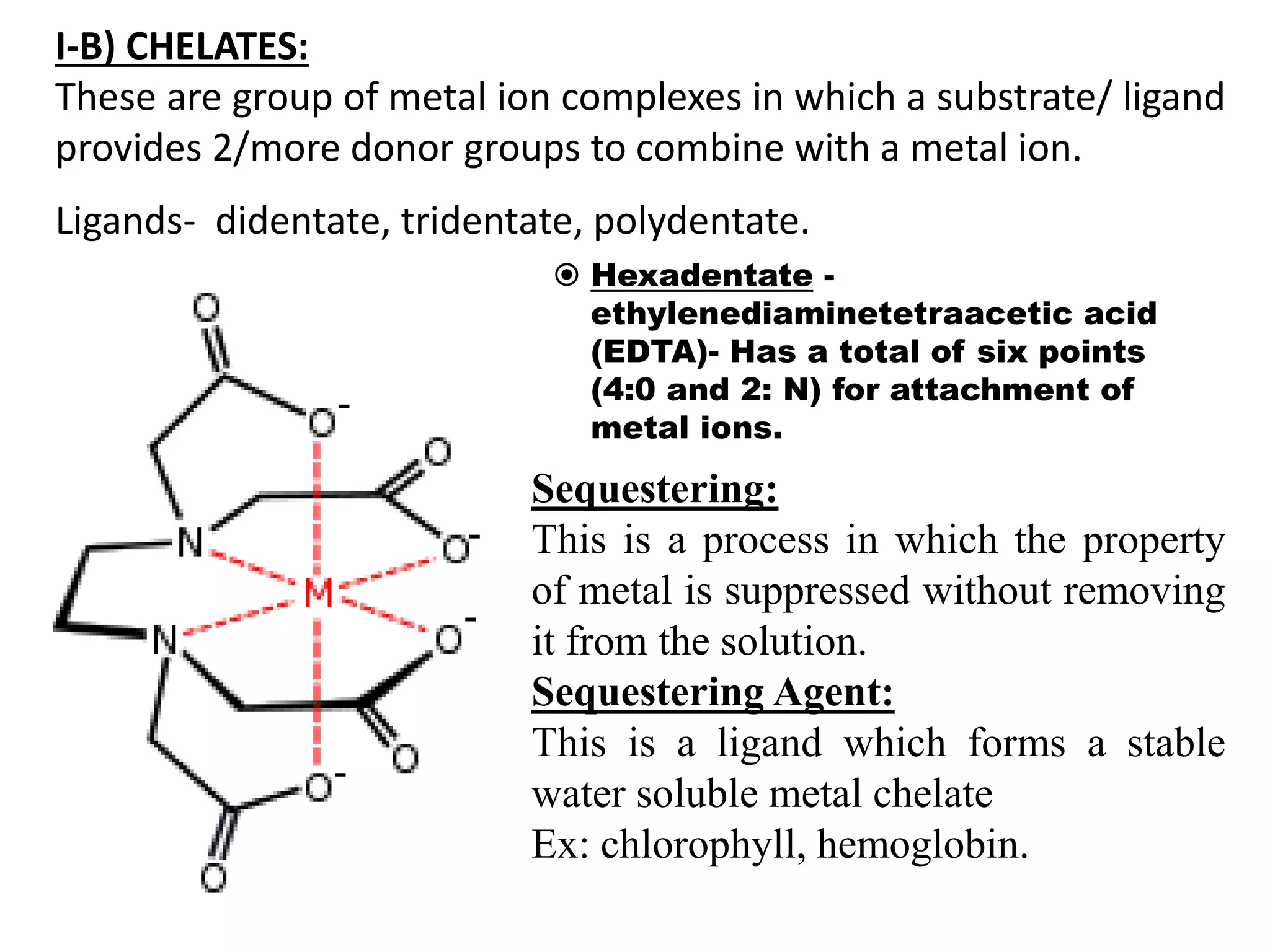
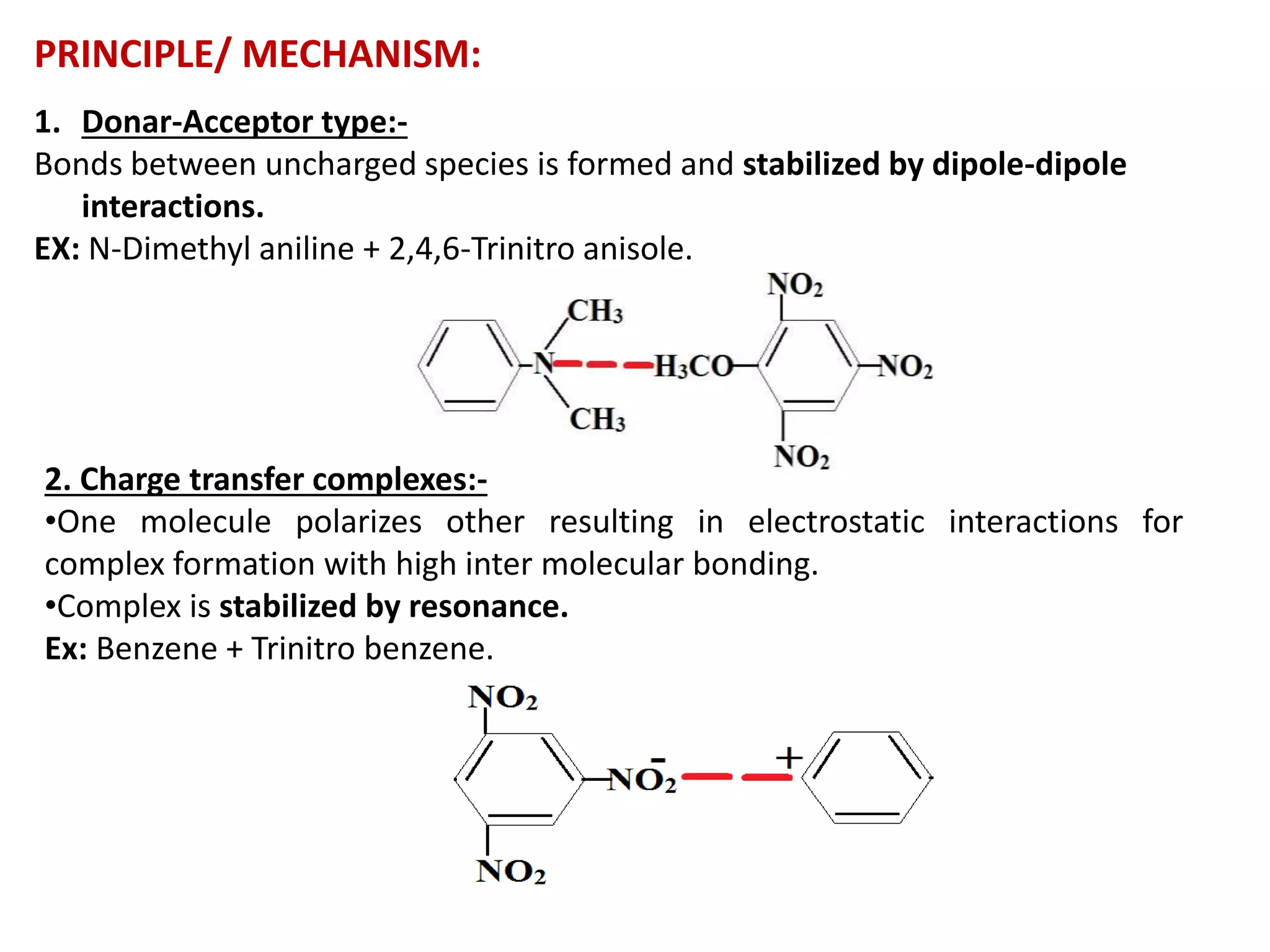
![I-A) INORGANIC COMPLEXES:
Werner postulates:
1. There are 2 types of valences primary (ionic), secondary
(coordinate).
2. Same type of anion/ radical/ molecule may be held by any one /
both type of valence.
3. Every central atom has fixed number of non-ionic valences (co-
ordination number)
4. The co-ordination atoms occupy the first sphere/coordination
sphere, other atoms occupy second/ ionization sphere.
5. Neutral molecules/ions may satisfy non-ionic valences.
6. The non-ionic valences are directed to specific positions in space.
Ex: [Co Cl (NH3)5] Cl2
Substrate Coordination sphere
Ionization sphere](https://image.slidesharecdn.com/complexation-180817061837/75/Complexation-5-2048.jpg)
![Ex: [Co Cl (NH3)5] Cl2
1. Compound ionize to form [Co Cl (NH3)5]+2 and 2Cl- .
2. Central chlorine do not precipitate with silver nitrate.
3. Substrate and ligand are bonded with coordination bond.
4. Coordination number is maximum number of atoms and groups
that combine with central atom in coordination sphere.
5. Co-ordination number for cobalt is 6.
Participate in
complexation](https://image.slidesharecdn.com/complexation-180817061837/75/Complexation-6-2048.jpg)












![General procedure:
Equation for complexation
M + n A MAn
Stability constant
Applying Log on both sides
Log [MAn] = log K + log [M] + n log [A]](https://image.slidesharecdn.com/complexation-180817061837/75/Complexation-27-2048.jpg)


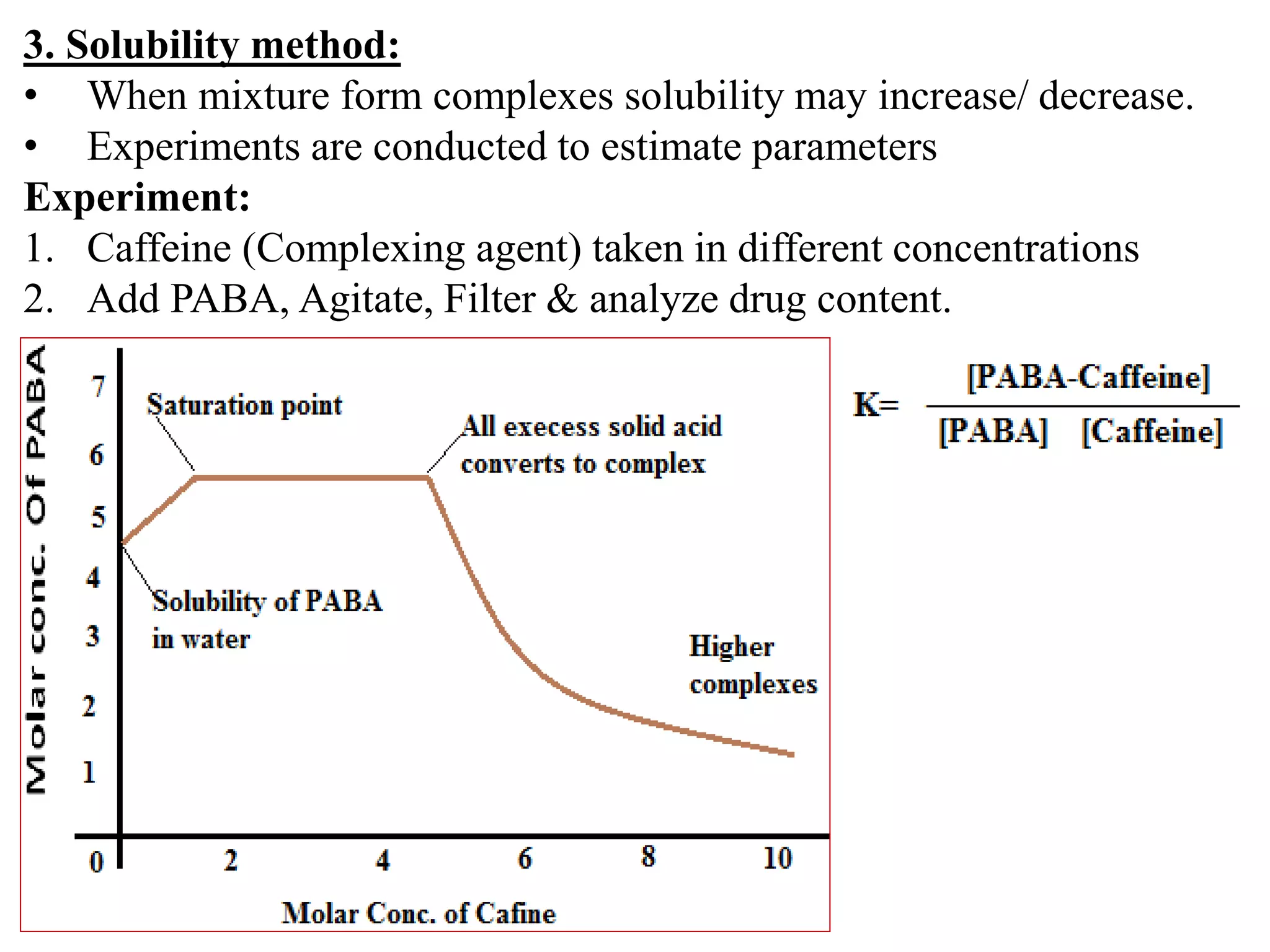
![4. pH titration method.
This method is suitable if complexation produces change in pH.
Experiment:
1. Glycine solution (75 ml) titrated with NaoH, pH is recorded.
2. Glycine solution (75 ml) + Cu+2 Complex titrated with NaoH,
pH is recorded. (Complexation releases Protons and pH decreases)
Quantity of alkali = Concentration of ligand bound.
Stability constant
Log β = 2 X p [A]
p [A] = pKa-pH-
log([HA]initial-[NaoH] )](https://image.slidesharecdn.com/complexation-180817061837/75/Complexation-32-2048.jpg)
