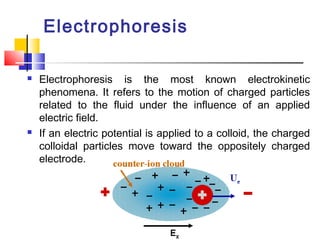
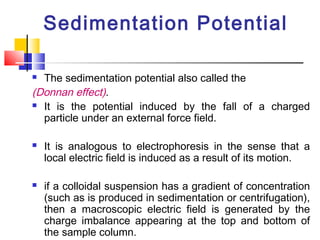
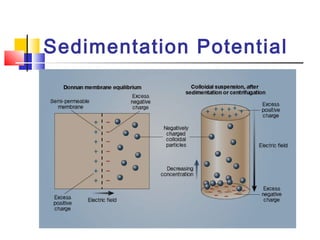
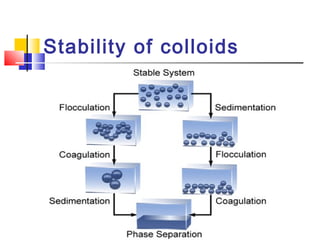
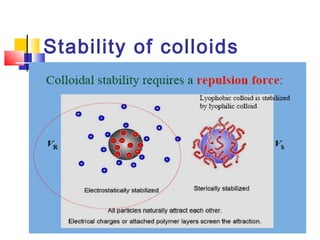
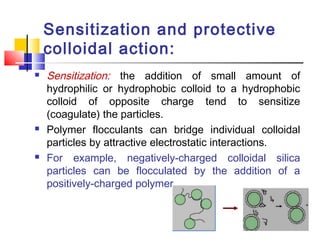
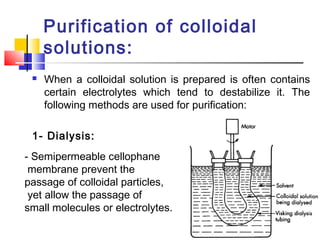
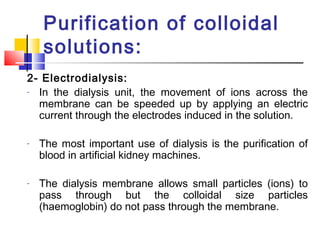
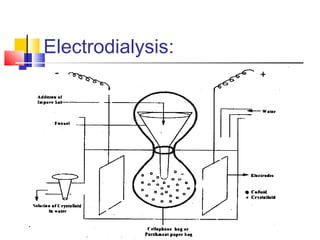
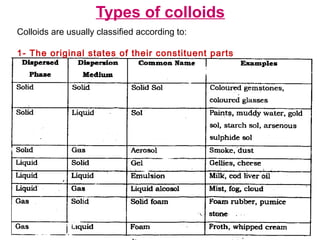
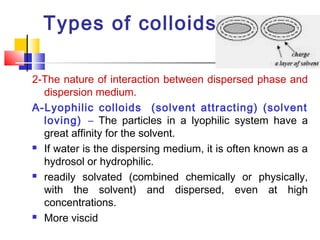
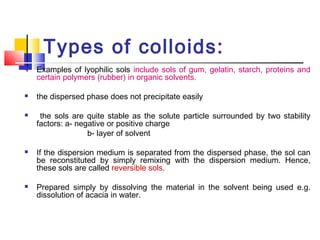
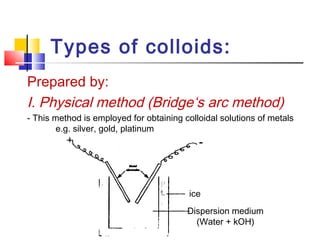
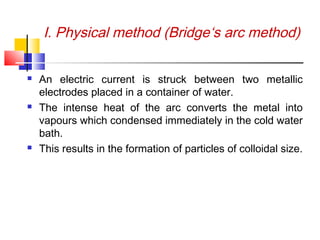
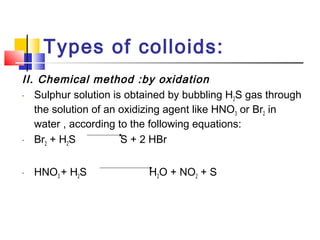
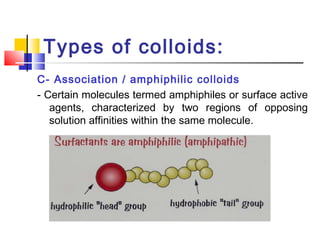
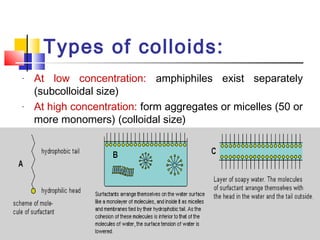
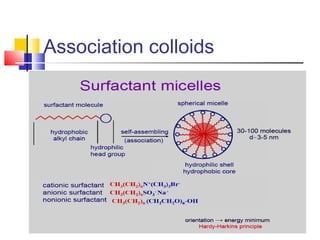
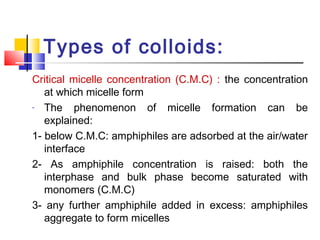
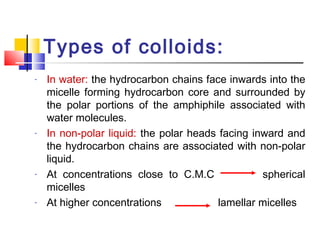
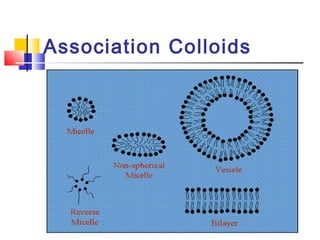
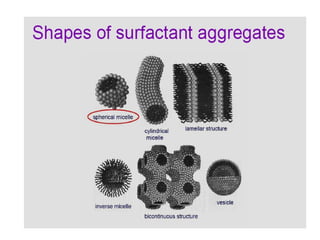
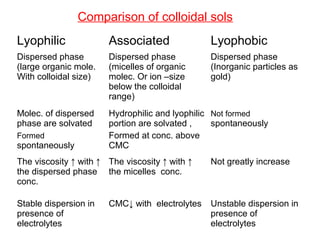
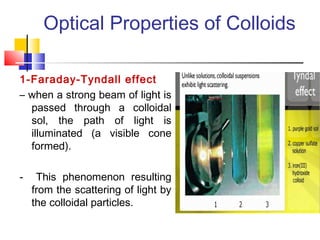
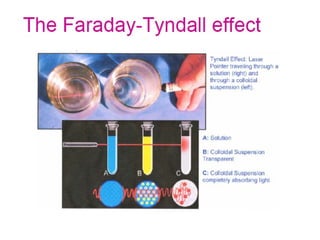
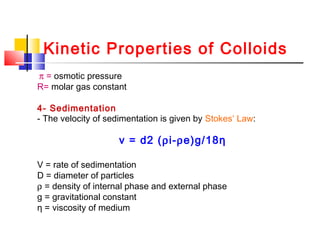
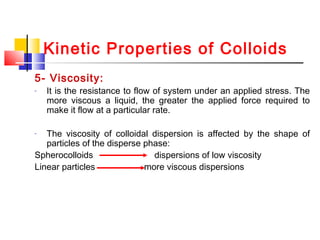
Colloids have particle sizes between 1 nm and 1000 nm. They are classified as lyophilic, lyophobic, or association colloids based on particle interactions with the dispersion medium. Lyophilic colloids readily disperse in the medium while lyophobic colloids do not. Association colloids form micelles above a critical micelle concentration. Colloids demonstrate optical properties like Tyndall effect and can be imaged with electron microscopes. They also exhibit kinetic properties including Brownian motion, diffusion, osmotic pressure, and sedimentation. Colloidal particles are often electrically charged, leading to electrokinetic phenomena like electrophoresis and electroosmosis. Stability is important for preventing









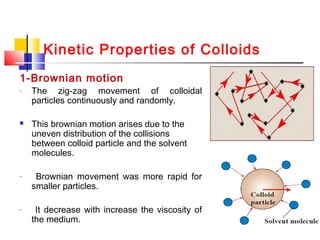

![Electric Properties Of
Colloids
Fe(OH)3 is positively charged
Due to self dissociation and loss of OH-
to the medium,so
they become [Fe(OH)3] Fe+3](https://image.slidesharecdn.com/colloids-171025110841/85/Colloids-45-320.jpg)