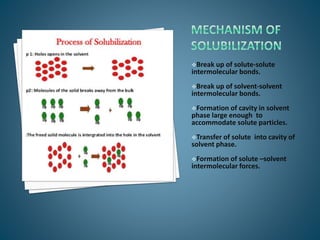
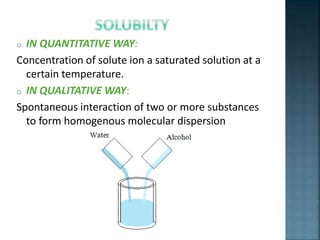
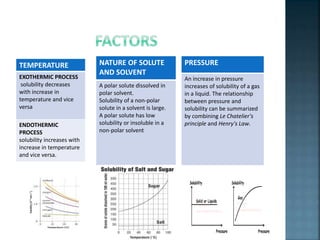
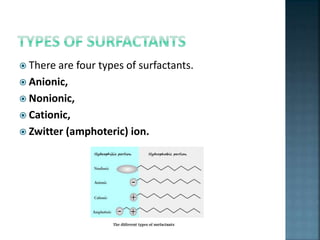
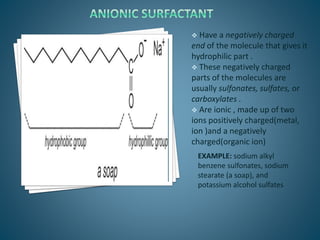
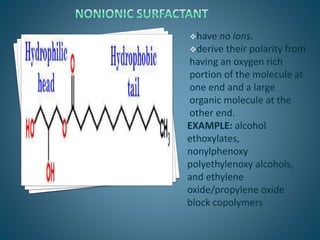
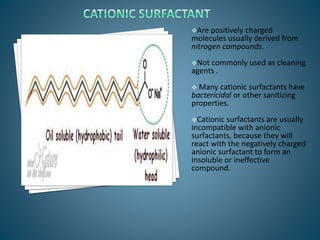
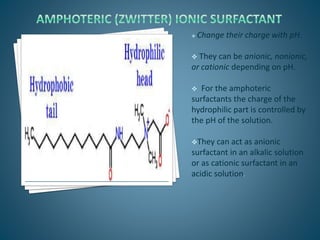
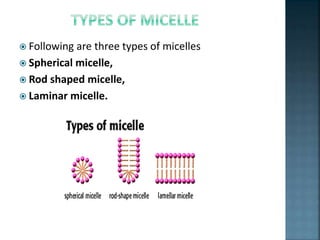
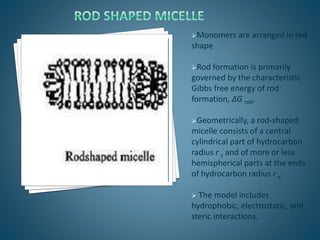
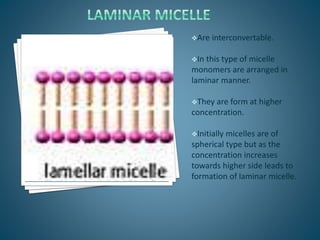
The document discusses the process of solubilization, including factors affecting solubility such as temperature, nature of solute or solvent, and pressure, while detailing the properties and types of surfactants which serve as wetting agents. It elaborates on amphipathic molecules, micelle formation in various environments, and describes different types of micelles including spherical, rod-shaped, and laminar micelles. Additionally, the document explains the behavior of surfactants and their interactions in different systems.