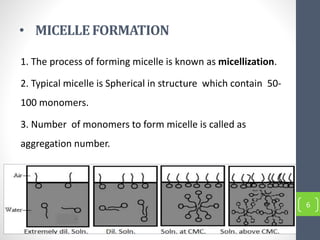
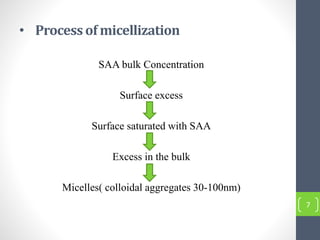
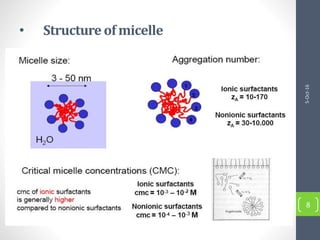
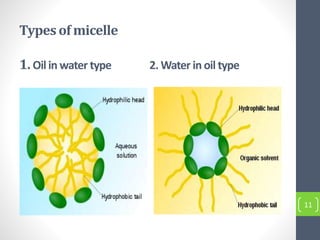
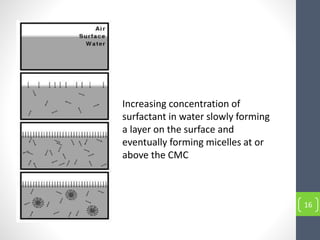
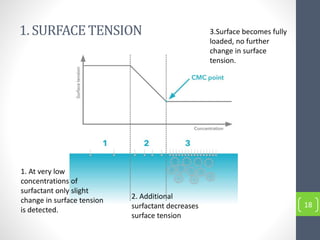
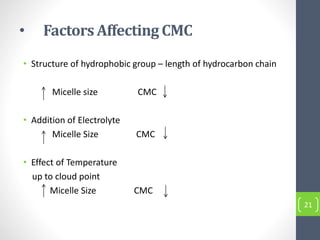
Critical micelle concentration refers to the minimum concentration of surfactant above which micelles start to form spontaneously in a solution. It is an important characteristic of surfactants that can be determined through measuring changes in properties like surface tension, conductivity, and turbidity at varying concentrations. Several factors influence the critical micelle concentration, including the structure of the surfactant's hydrophobic group and the presence of electrolytes in the solution.