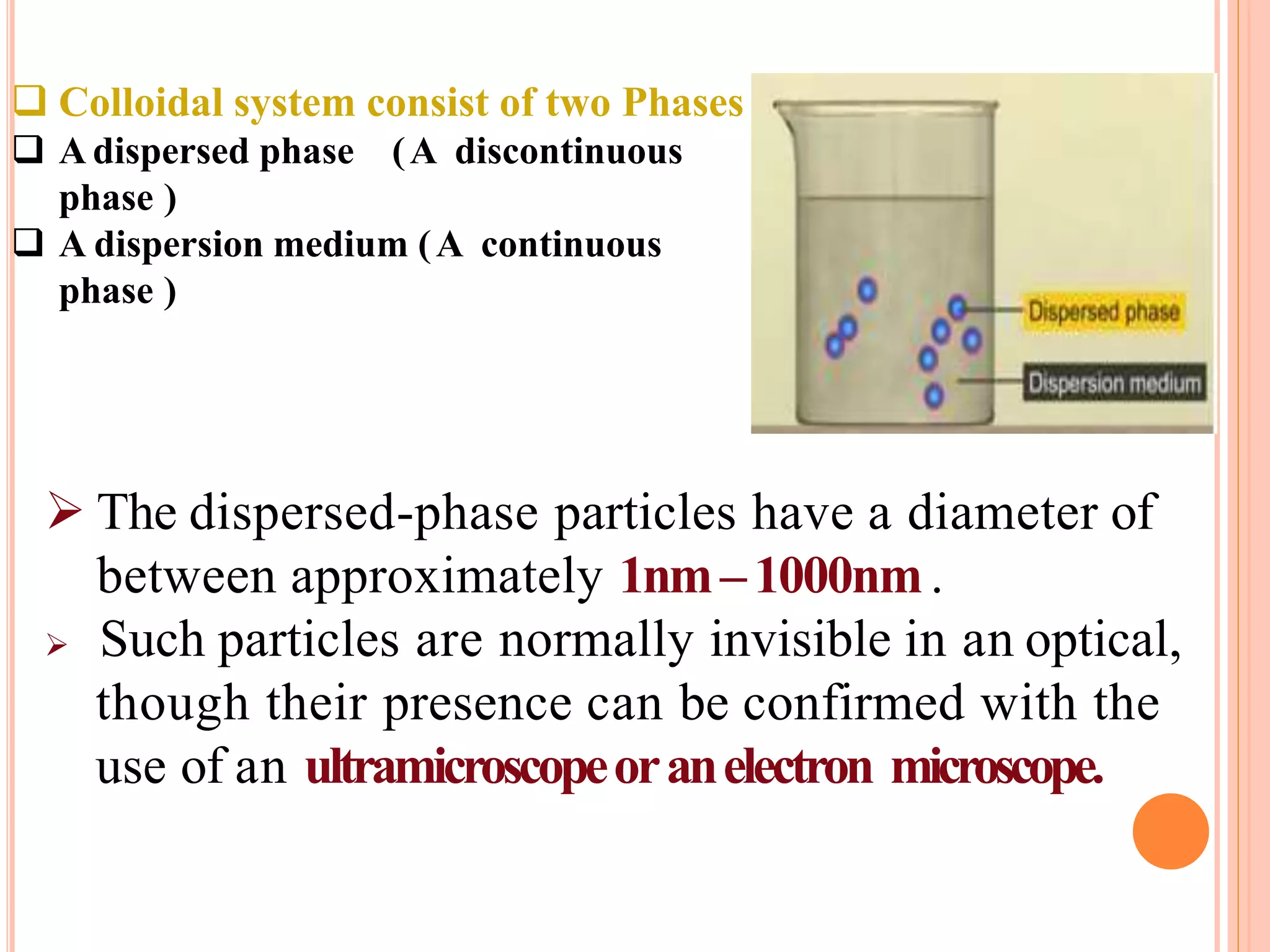
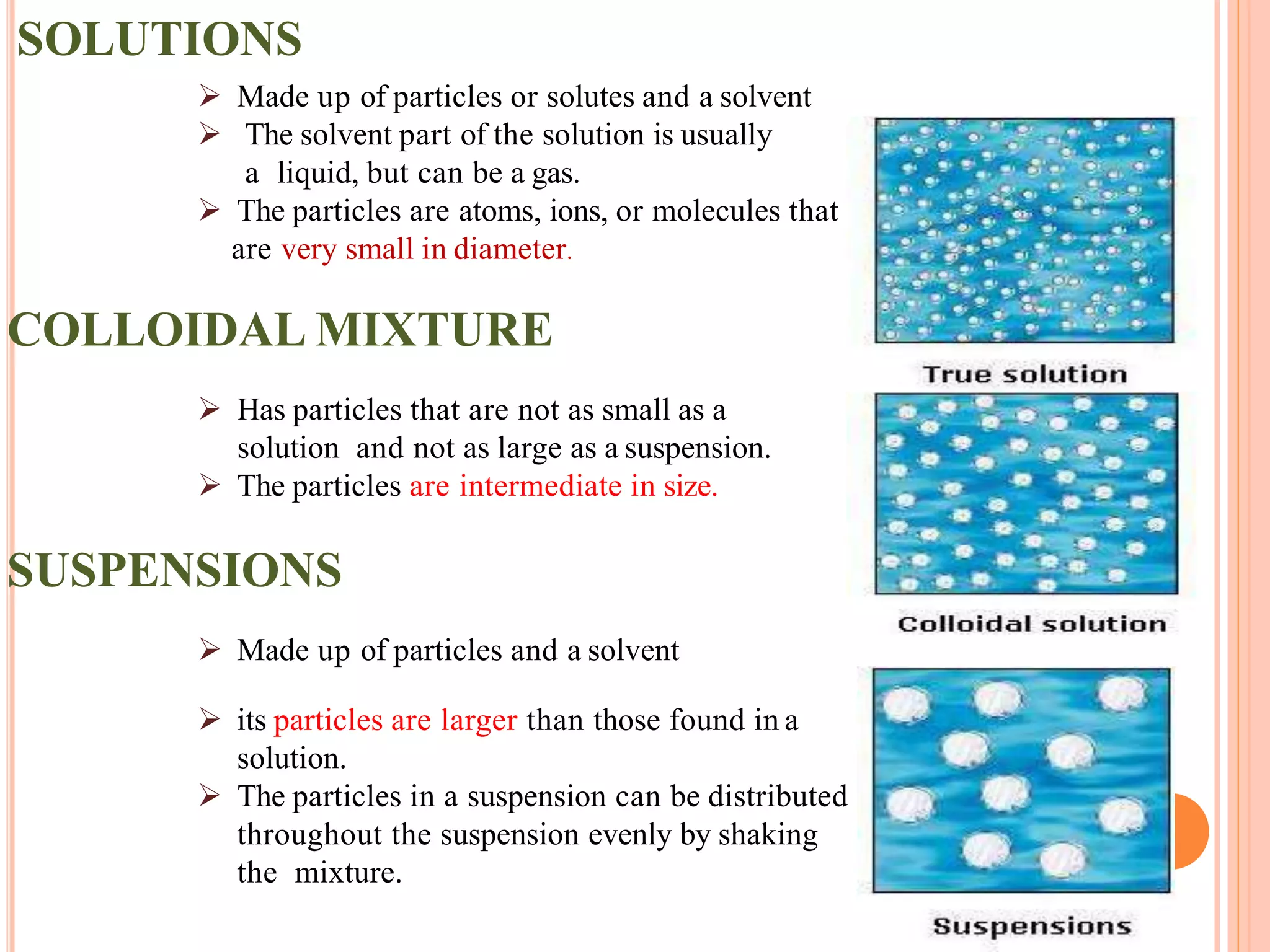
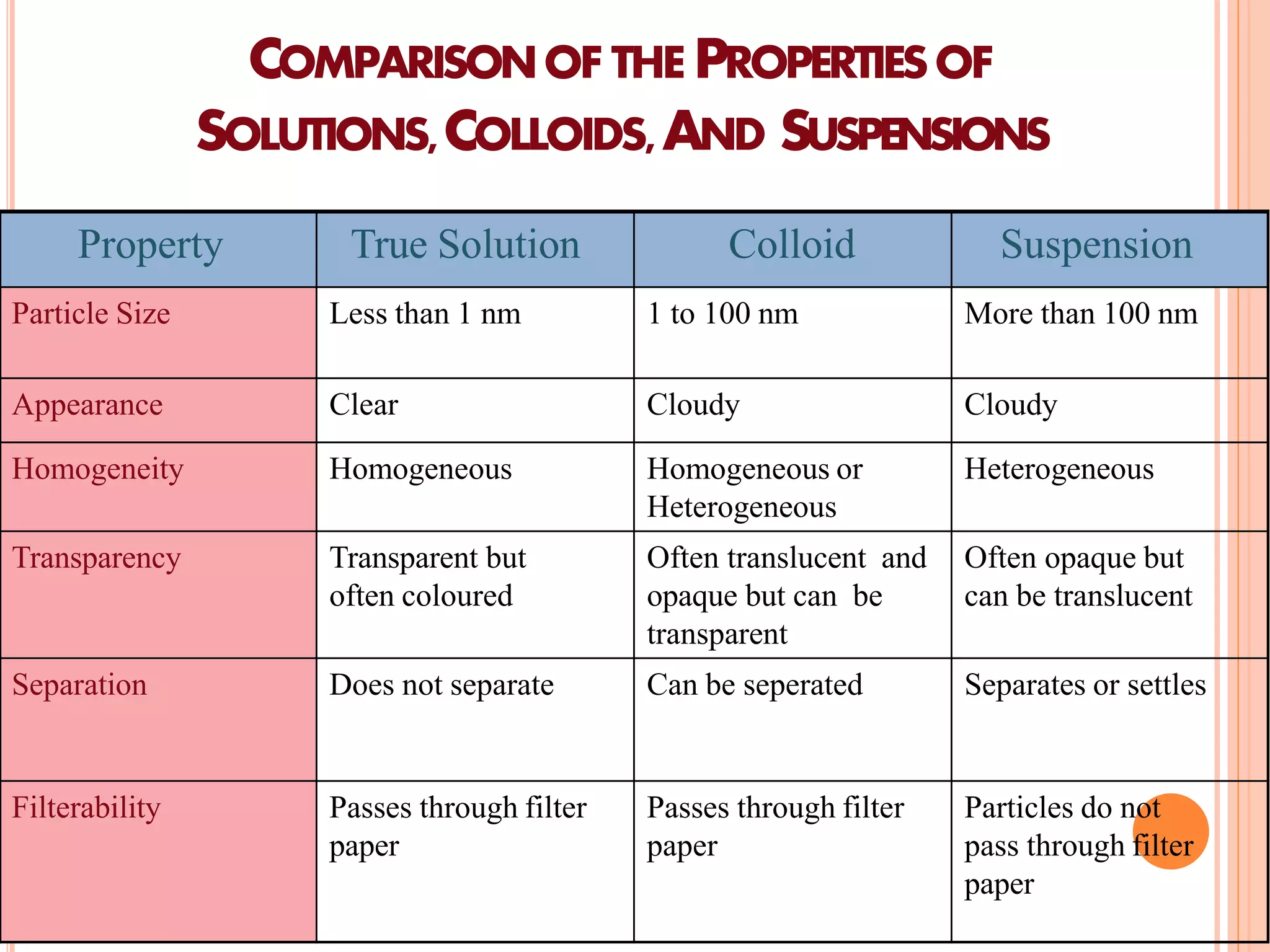
This document provides information about colloidal dispersions. It defines a colloid as a substance microscopically dispersed throughout another substance, with particle sizes between 1-1000nm. Colloids can be classified based on their physical state, nature of interactions, size, appearance, or electric charge. Key properties of colloids include Brownian motion, diffusion, sedimentation, viscosity, light scattering, and electrical behaviors like electrophoresis and electrosmosis. Colloids find applications in areas like therapy, absorption, solubility, stability, and drug targeting.