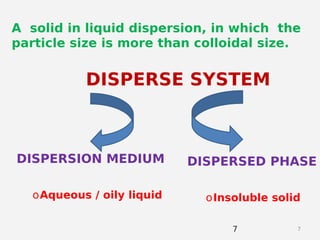
This document discusses coarse dispersion suspensions. It defines suspensions as heterogeneous systems with two phases, a solid dispersed phase and a liquid continuous phase. The key points covered include:
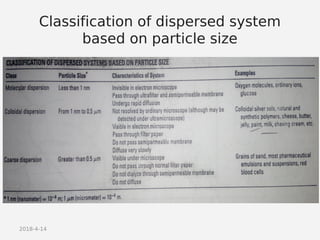
- Classifying suspensions based on particle size as coarse, colloidal, or molecular dispersions.
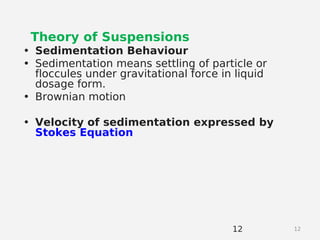
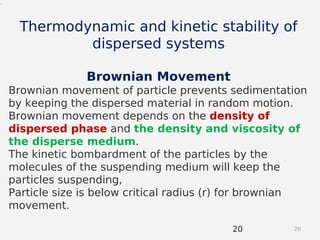
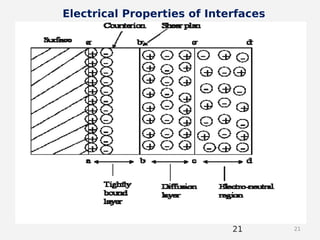
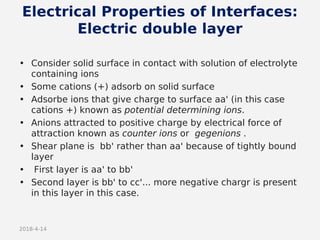
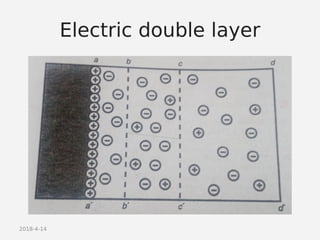
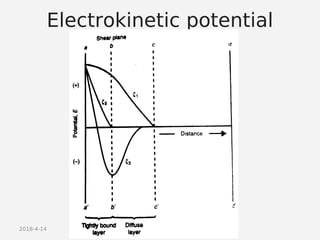
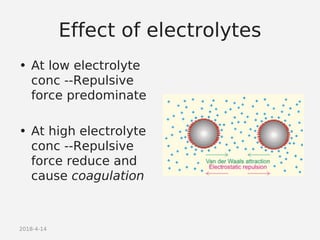
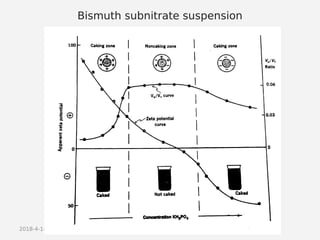
- Theories behind sedimentation behavior, Brownian motion, and electrokinetic properties that impact suspension stability.
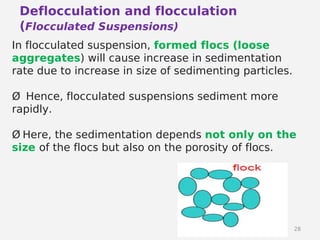
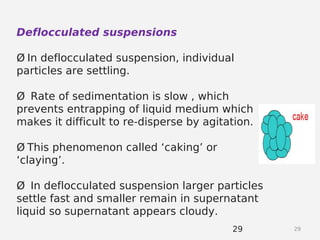
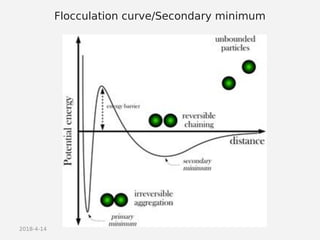
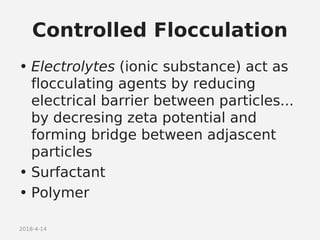
- Factors that influence flocculation vs deflocculation like zeta potential, electrolyte concentration, and addition of surfactants or polymers.
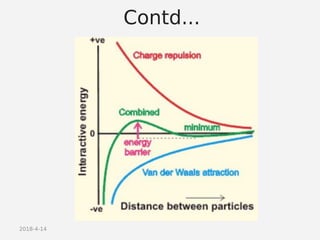
- DLVO theory explaining the balance of attractive van der Waals forces and repulsive electrostatic forces between particles.
- How temperature changes can impact physical