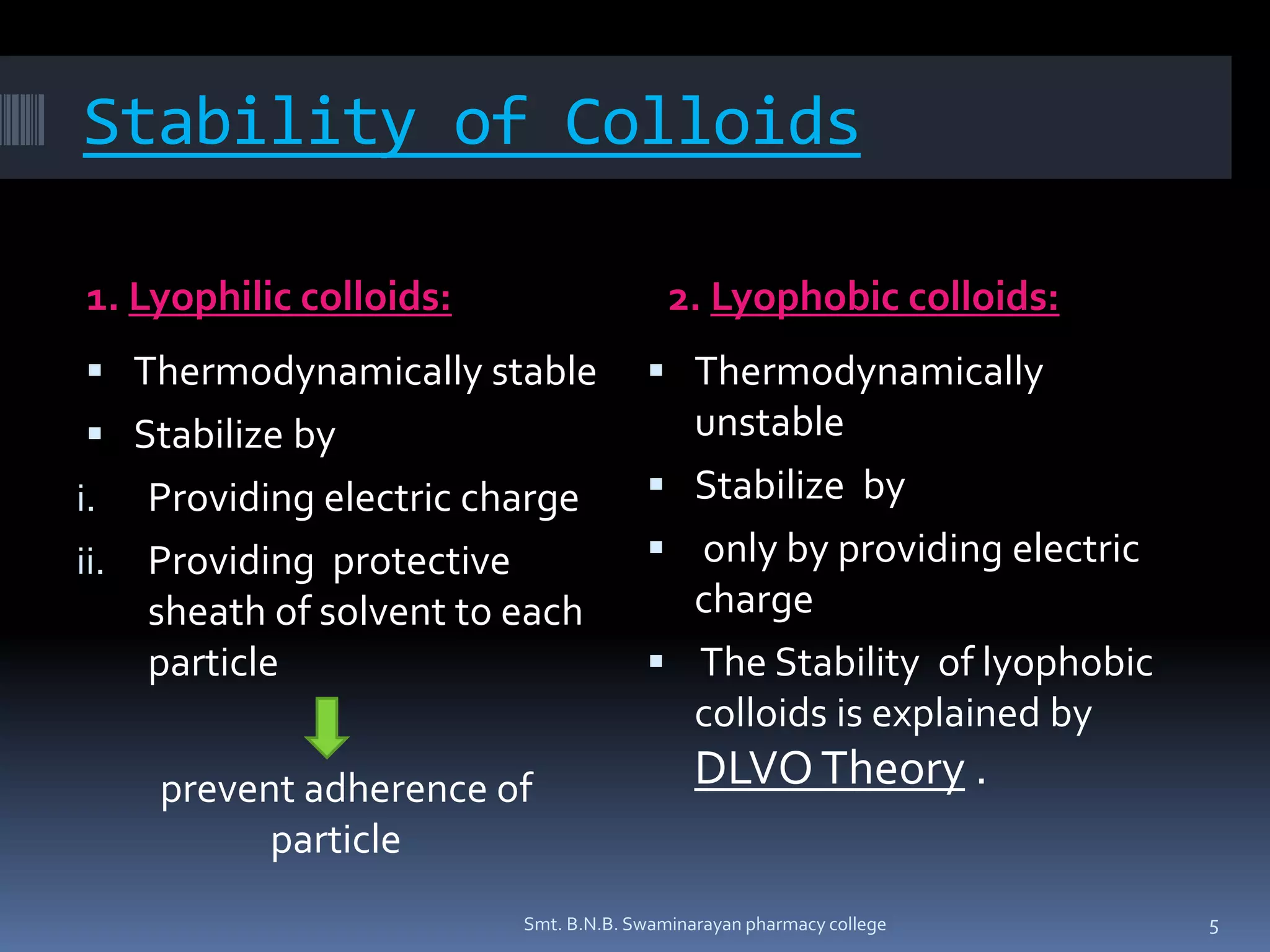
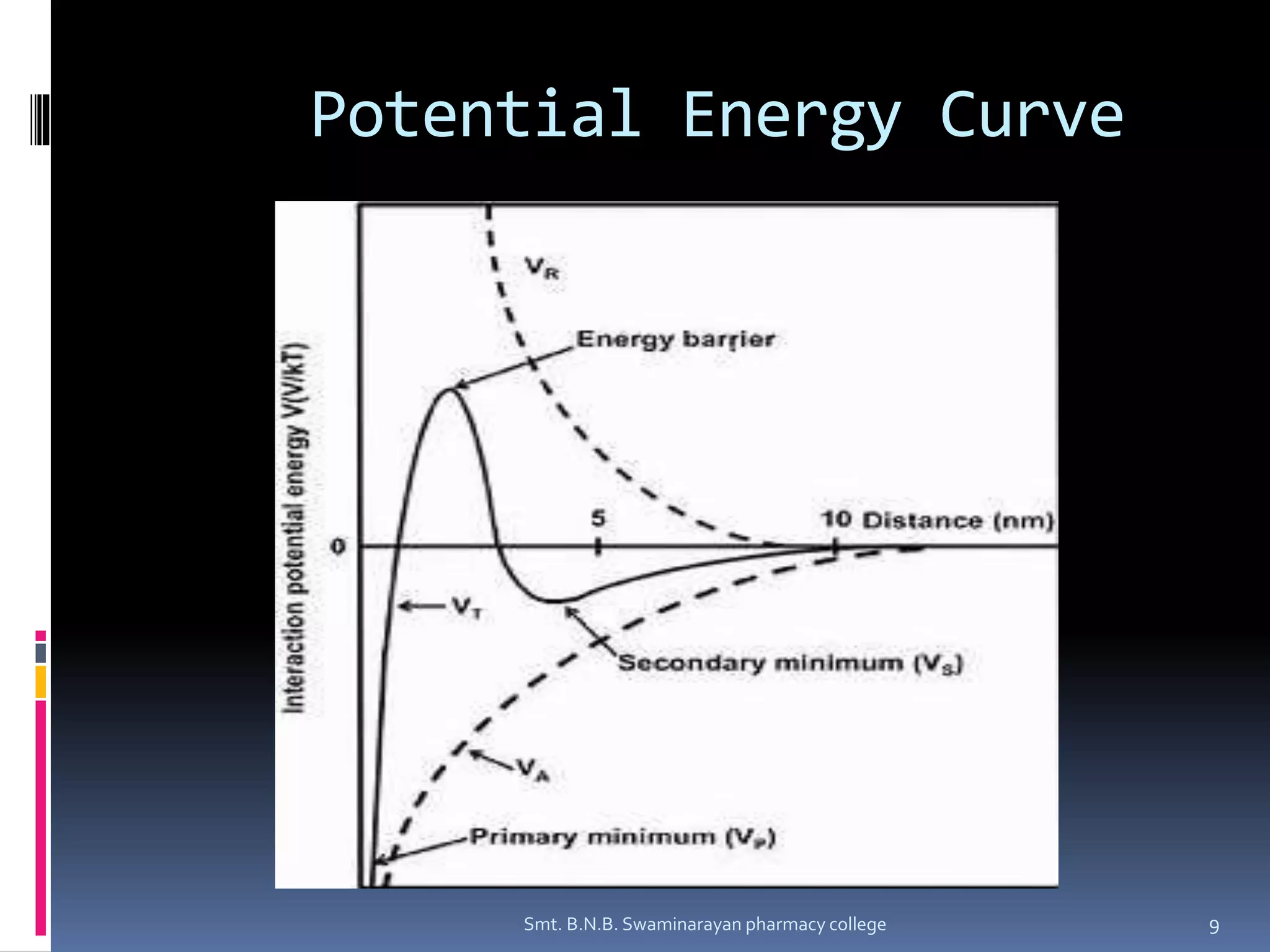
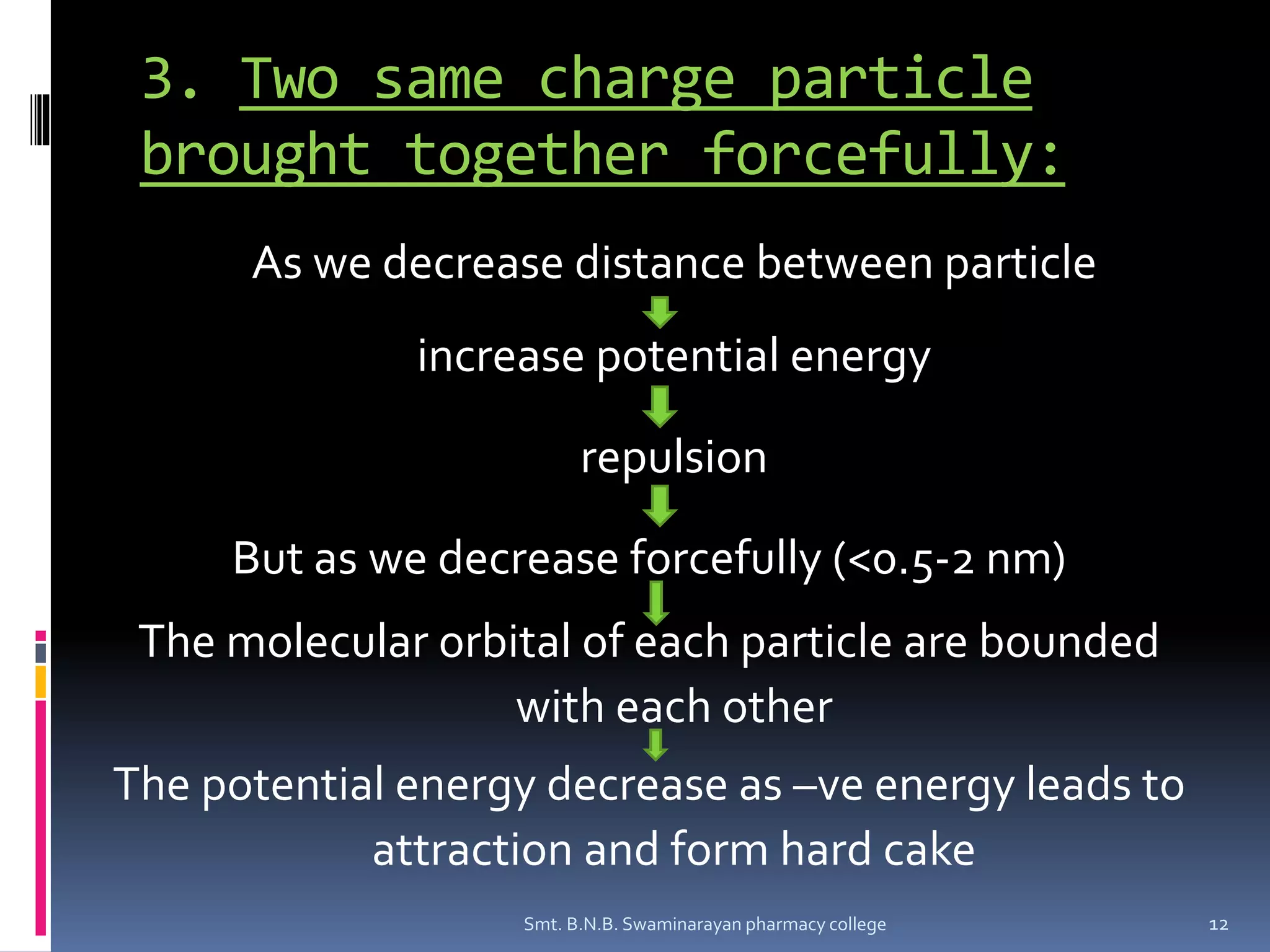
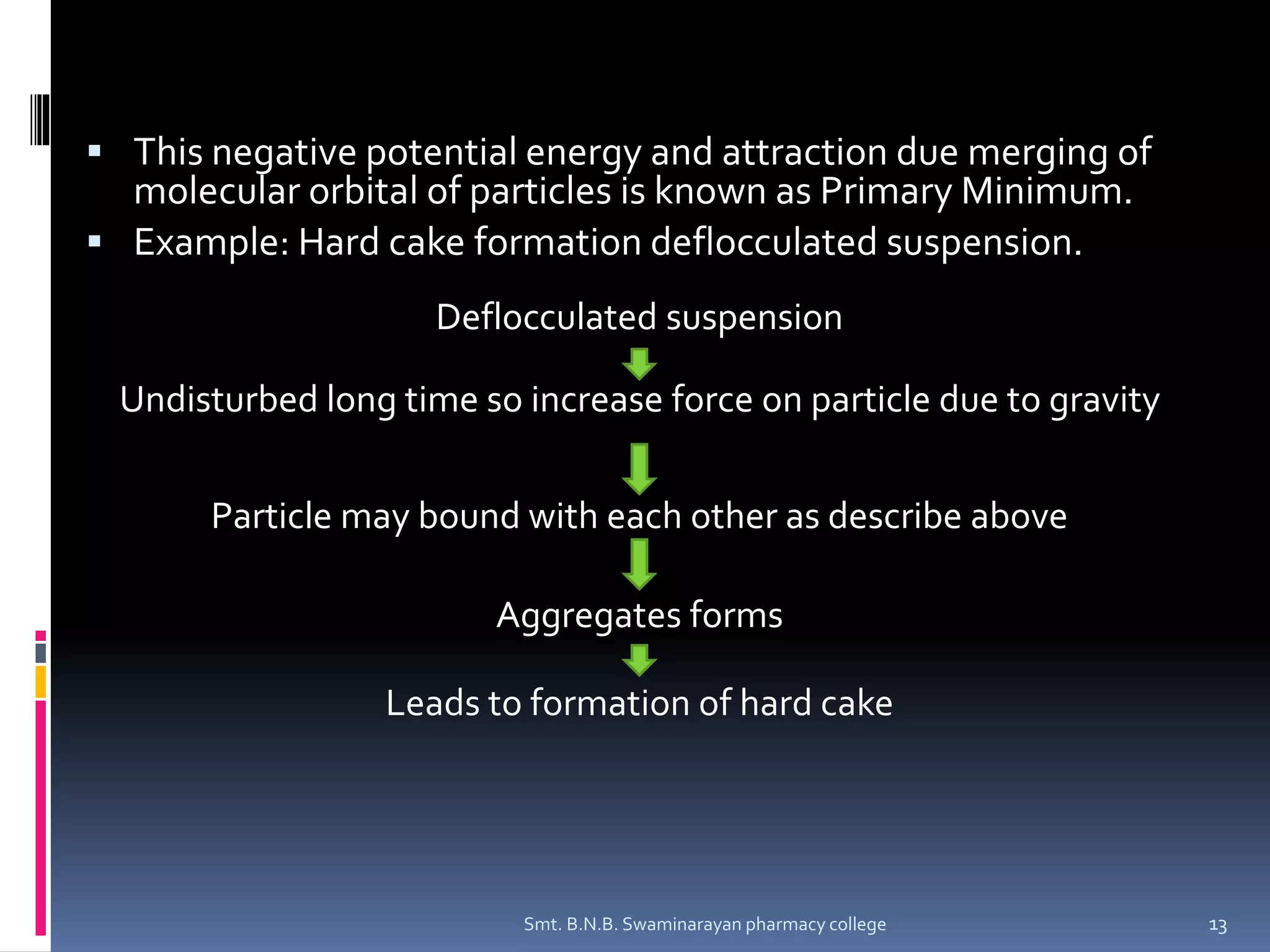
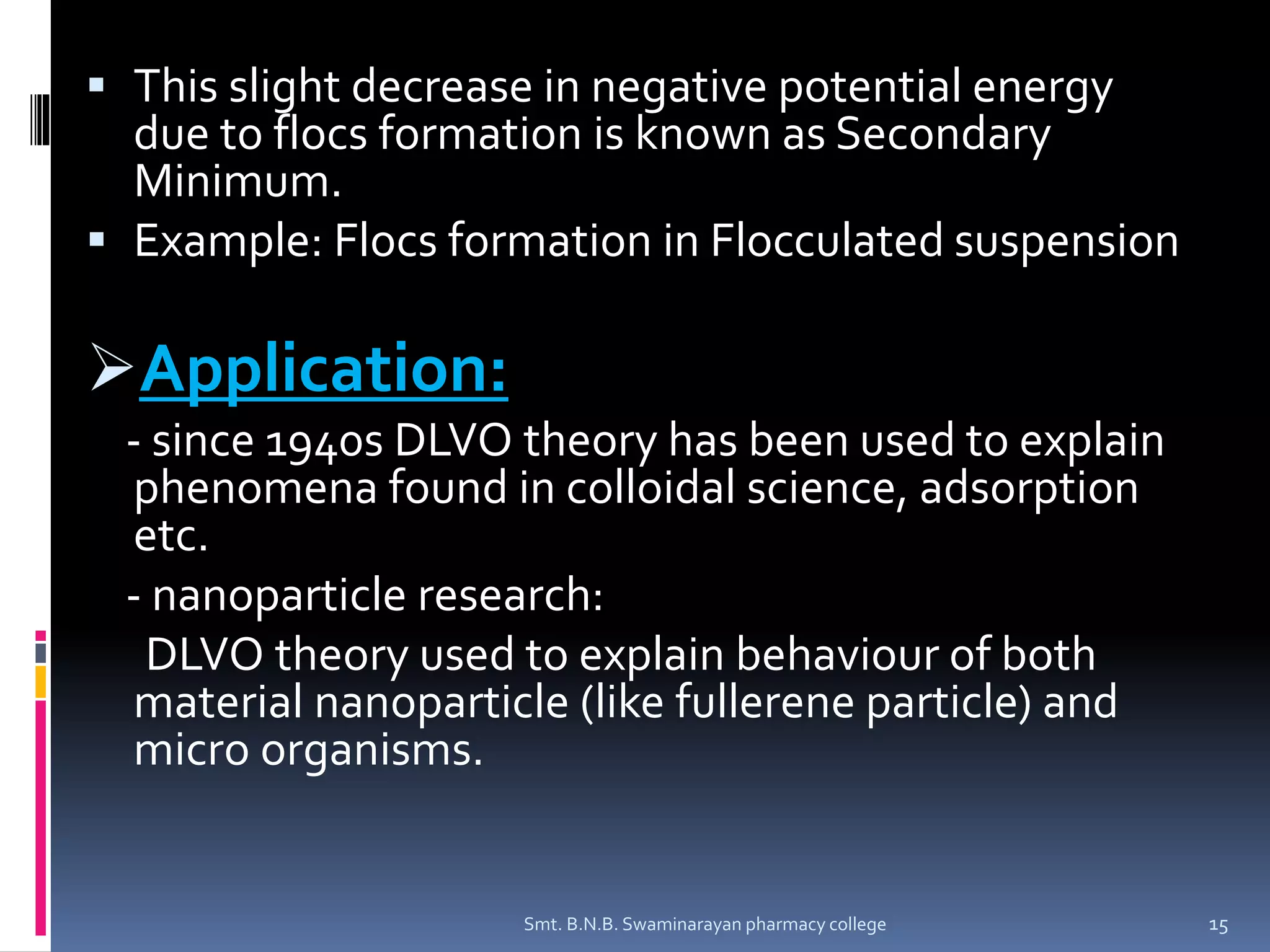
The document discusses the stability of colloids, specifically focusing on lyophobic colloids and the DLVO theory, which explains the forces acting on colloidal particles in a dispersion medium due to electrostatic repulsion and van der Waals attraction. It classifies colloids based on the nature of their interaction with solvent and explains how zeta potential affects stability in suspensions, highlighting concepts like deflocculated and flocculated suspensions. Additionally, the document references applications of DLVO theory in colloidal science and nanoparticle research.