Embed presentation
Download as PDF, PPTX





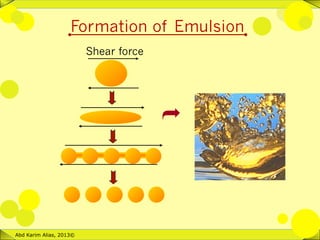



An emulsion consists of two immiscible liquids, like oil and water, with one dispersed as small spherical droplets in the other. There are two types of simple emulsions: water-in-oil and oil-in-water. Food emulsions are usually stabilized by emulsifiers and have droplet sizes ranging from 0.1 to 100 micrometers. The formation of emulsions requires the dispersion of one liquid phase into small droplets through processes like homogenization, which increases the interfacial area between the phases.







