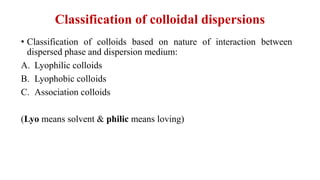
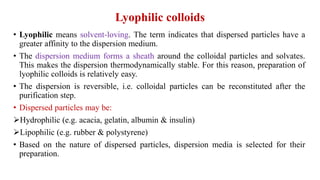
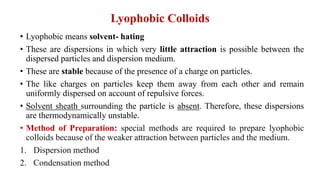
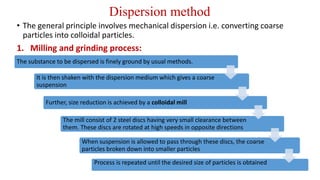
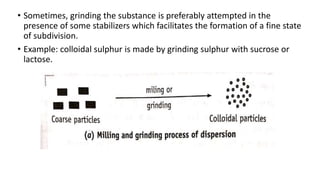
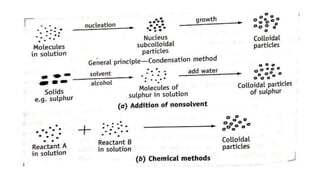
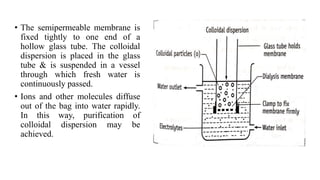
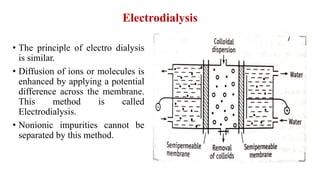
This document discusses the classification and preparation of colloidal dispersions. It begins by classifying colloids based on the interaction between the dispersed phase and dispersion medium into lyophilic, lyophobic, and association colloids. Lyophilic colloids have affinity for the dispersion medium, making them thermodynamically stable. Lyophobic colloids require special preparation methods since the dispersed particles are solvent-hating. Association colloids involve micelle formation using surfactants above the critical micelle concentration. The document also describes various methods for preparing and purifying colloidal dispersions, including mechanical grinding, peptization, addition of nonsolvents, and ultrafiltration.