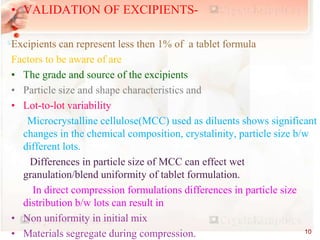
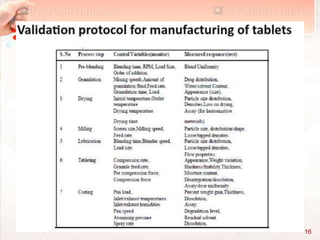
This document discusses validation of solid dosage forms such as tablets. It defines validation as establishing documented evidence that a process will consistently produce products meeting specifications. The major types of validation are prospective, concurrent, and retrospective. Product validation involves validating raw materials, analytical methods, equipment, process parameters, and finished product tests. Validation of tablets includes processes like mixing, wet granulation, drying, lubrication, compression, coating, and in-process and finished product testing. Process parameters that must be validated for each unit operation include equipment, material attributes, times, temperatures, and product quality tests.