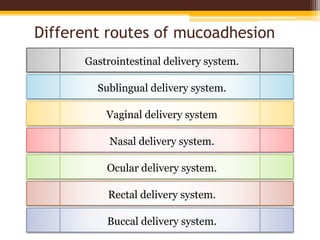
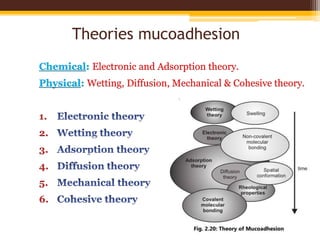
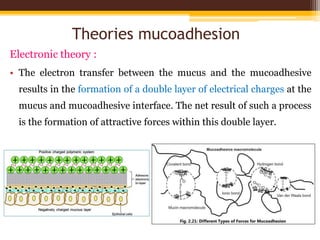
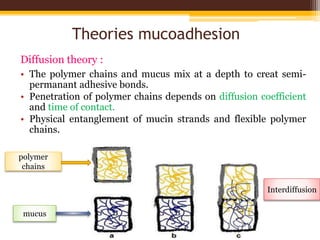
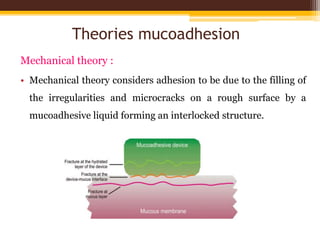
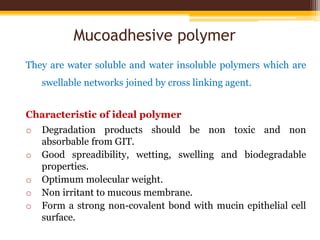
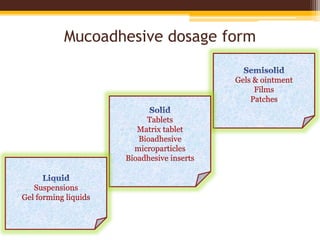
This document discusses mucoadhesive drug delivery systems (MDDS). It begins by defining MDDS as systems that use the bioadhesive properties of certain polymers to target and prolong the release of drugs at mucous membranes. It then covers the basics of mucous membranes and their structure, composition, and functions. The document discusses the need for MDDS to enhance drug absorption, prolong drug residence time, and target drug delivery. It also outlines the advantages and disadvantages of MDDS, various routes of administration, mechanisms of mucoadhesion, theories of mucoadhesion, mucoadhesive polymers, and methods of evaluating MDDS. In the end, it provides some applications of MDDS such as vaccine delivery, cancer