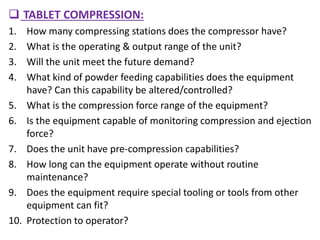
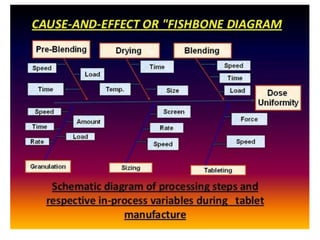
This document discusses process validation for solid dosage forms like tablets. It defines process validation and describes the types including prospective, retrospective, concurrent, and revalidation. It discusses evaluating tablet composition and selecting manufacturing processes. Key areas covered include mixing, wet and dry granulation, milling, compression, and coating. It also addresses evaluating equipment used in the processes. The goal of process validation is to consistently produce tablets meeting quality standards.