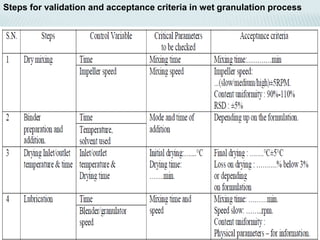
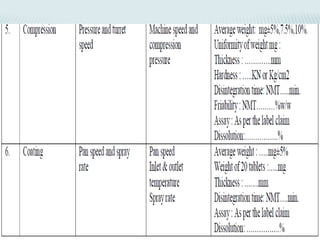
The document discusses validation of processing techniques for wet granulation. It outlines key parameters that must be considered and validated at each stage of wet granulation including blending, wet granulation, drying, and tablet compression. Parameters like mixing time and speed, binder concentration, granulation endpoint, drying temperature and moisture content, and compression force must be optimized and shown to consistently produce tablets meeting quality standards. Equipment used at each stage must also be validated to ensure it is suitable for the processing steps.