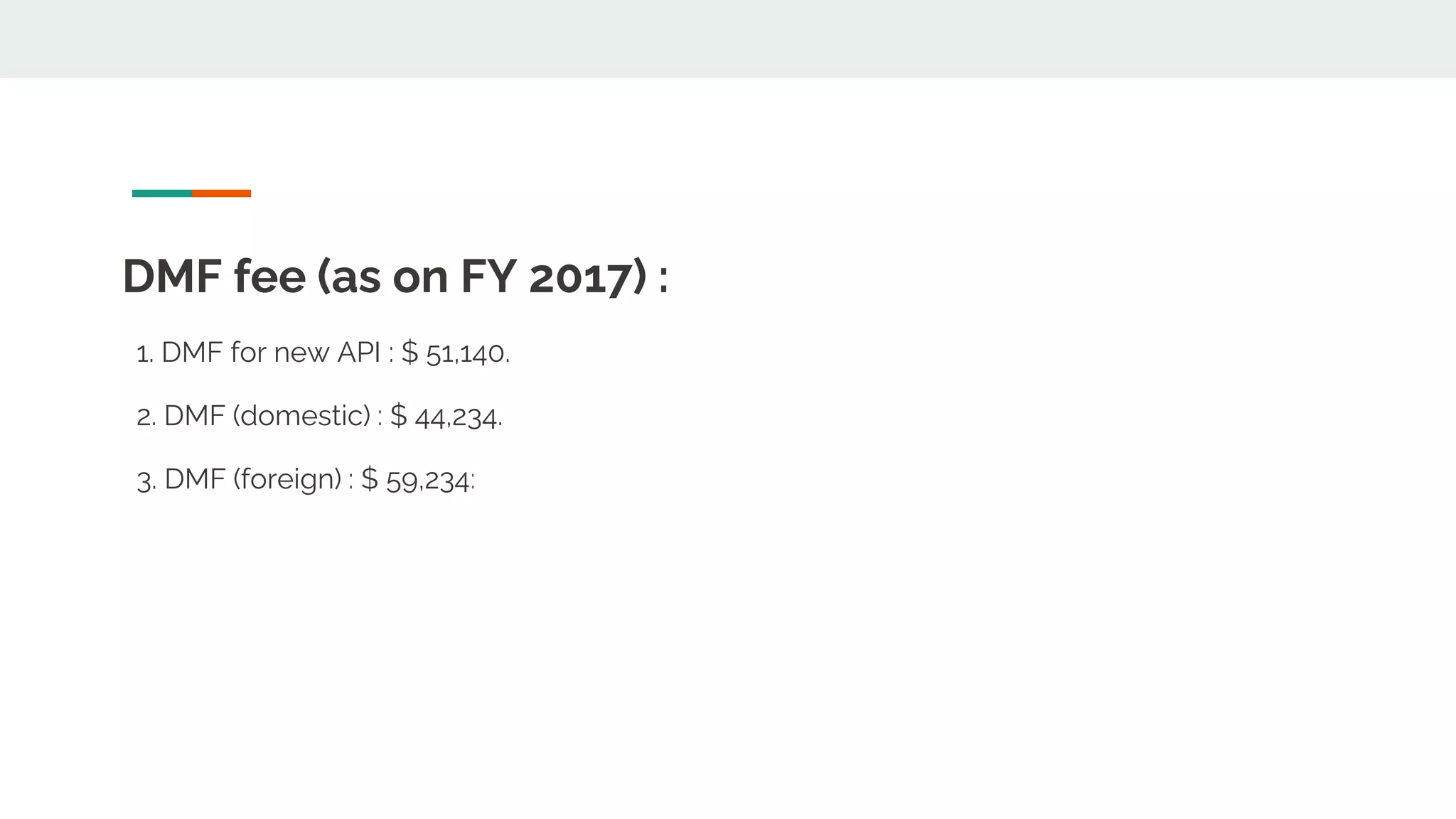
The document discusses regulatory requirements for registering an active pharmaceutical ingredient (API). It explains that API registration requires submitting a dossier containing information about the quality of the API. This includes details on manufacturing, characterization, controls, and stability data. The dossier is submitted to health authorities for marketing authorization. It also describes drug master files (DMFs), which provide confidential API information to regulators, and notes their use in the US and EU registration processes. DMFs can reference other DMFs. The document outlines the organization and sections of electronic common technical documents (eCTDs) used to submit API information digitally.