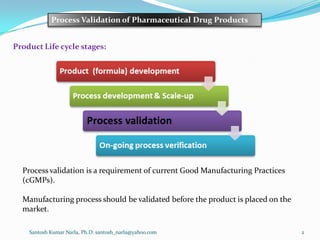
This document discusses process validation for pharmaceutical drug products. It defines process validation as documented evidence that a process can reproducibly produce a product meeting its specifications. Process validation is required by cGMP regulations and must be done before market approval. Traditional prospective validation establishes evidence before implementation, while continuous process verification monitors current production. Validation covers facilities, equipment, methods, staff training, and includes design space verification. The validation scheme submitted to regulators includes the manufacturing process description, in-process controls, acceptance criteria and reports.


![Santosh Kumar Narla, Ph.D: santosh_narla@yahoo.com 5
Process Validation of Pharmaceutical Drug Products
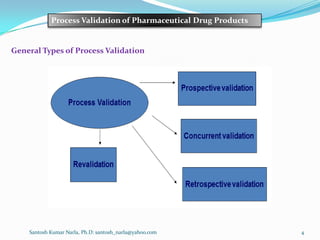
a) Prospective validation: [Traditional Process validation]
Establishing documented evidence prior to process implementation that a
system does what it proposed to do based on preplanned protocols.
It is completed prior to the distribution and sale of the medicinal product.
It is carried out during the development stage.
b) Concurrent validation: [Continuous process verification]
Validate processes during routine production.
Establishing documented evidence that a facility and processes do what they
purport to do, based on information generated during actual imputation of the
process.
This approach involves monitoring of critical processing steps and end product
testing of current production, to show that the manufacturing process is in a
state of control.](https://image.slidesharecdn.com/processvalidationofdrugproduct-150417111927-conversion-gate01/85/Process-validation-of-drug-product-5-320.jpg)