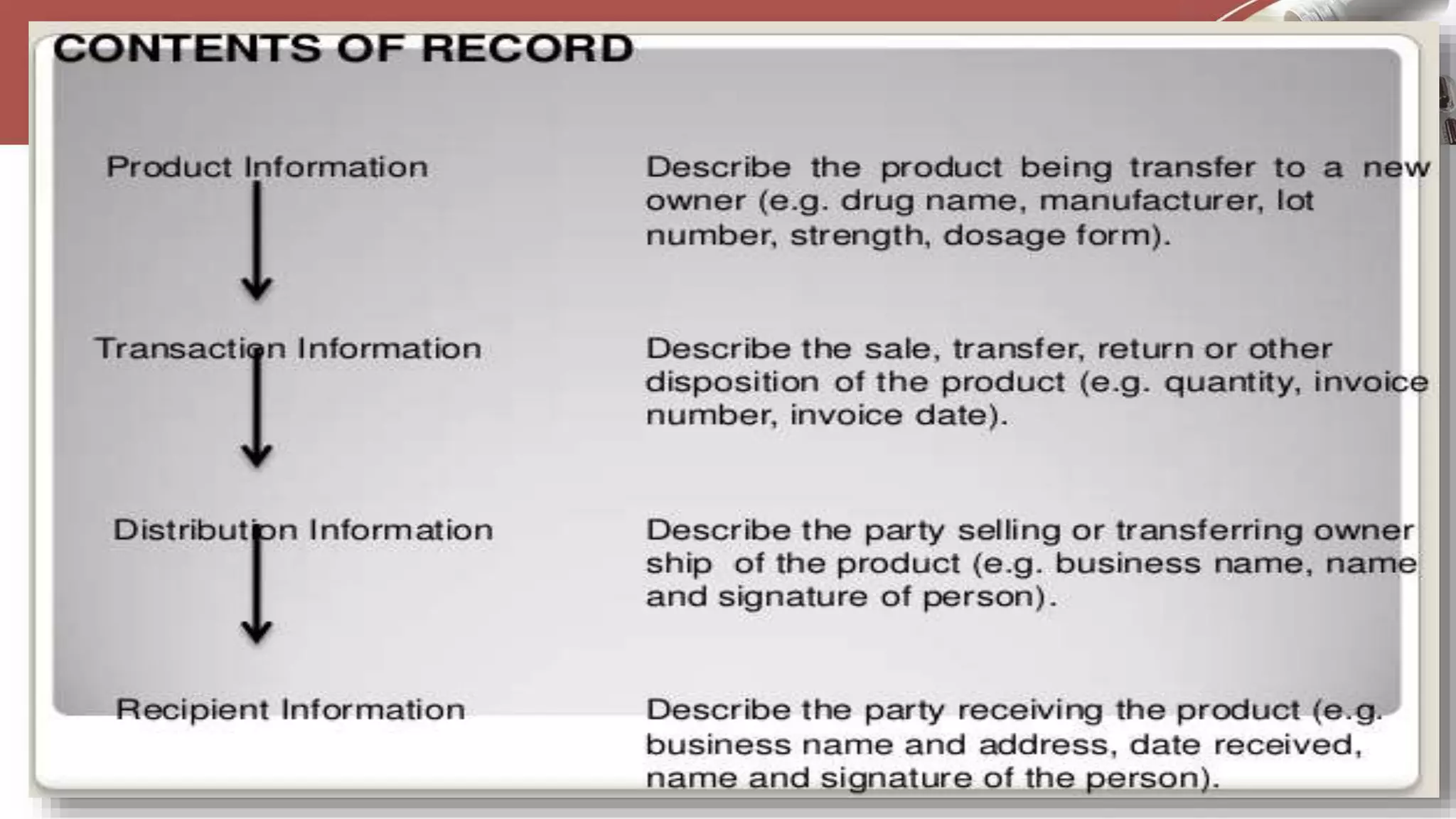
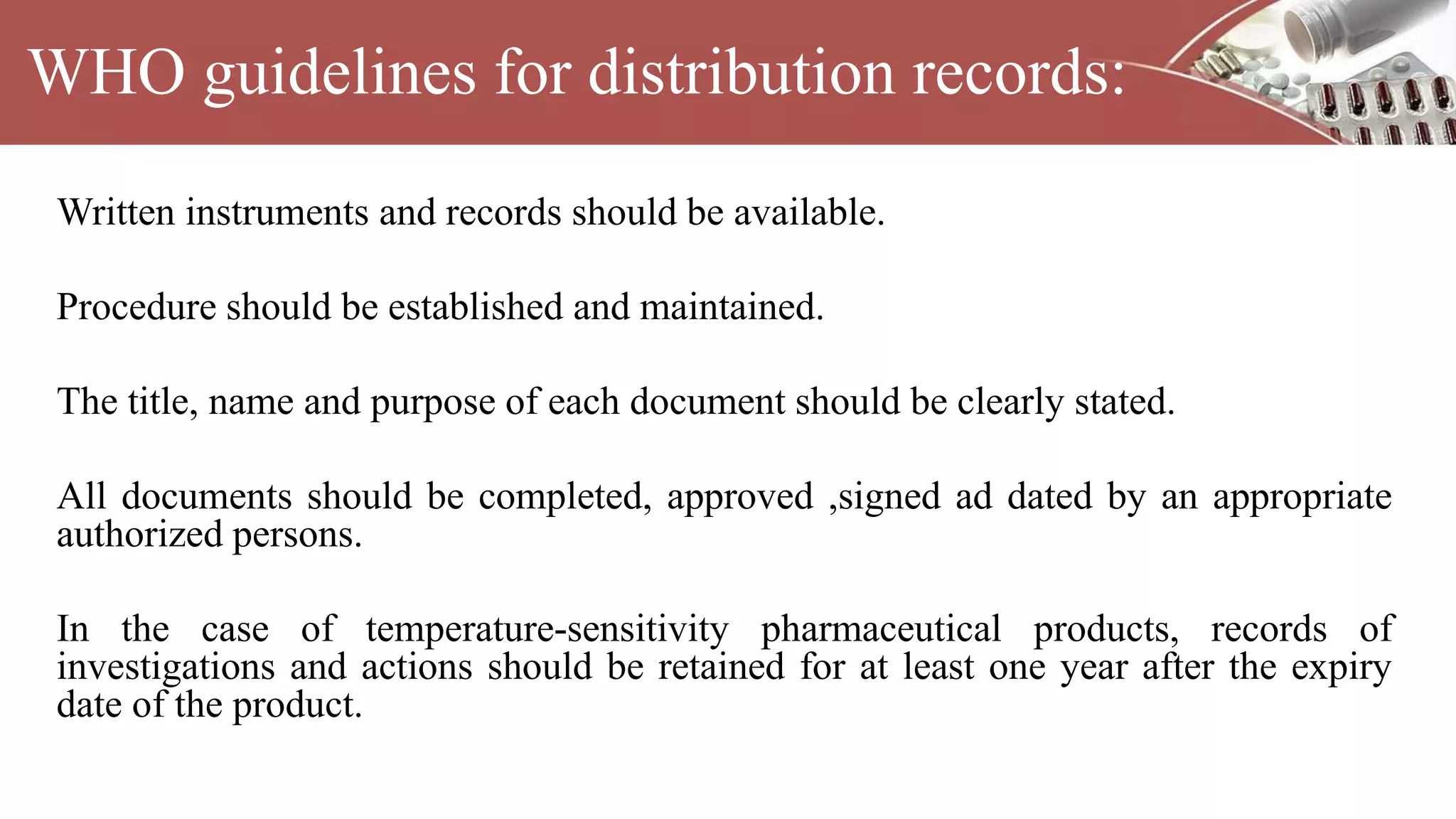
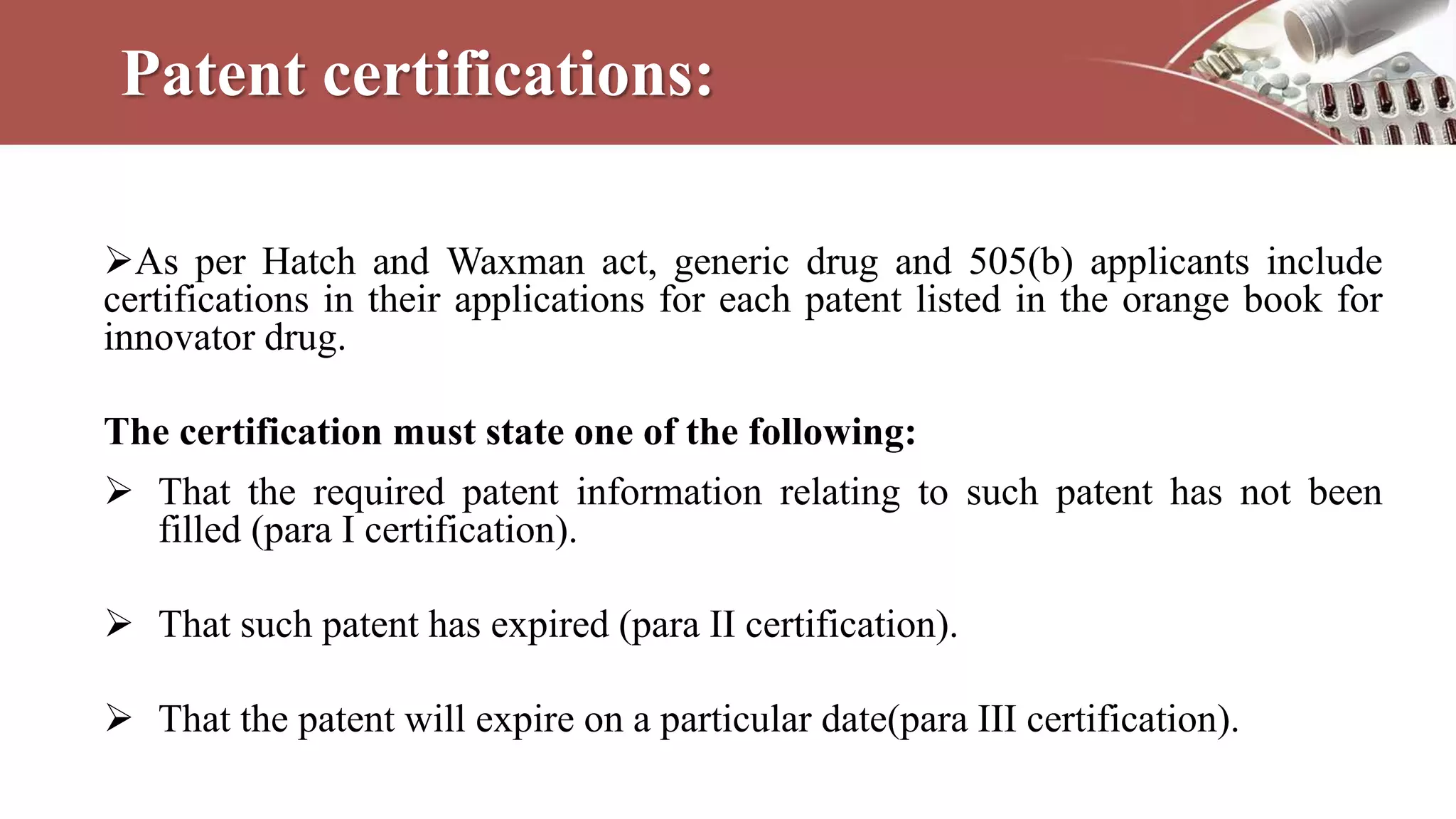
This document discusses various types of documentation required in the pharmaceutical industry. It begins by explaining that documentation provides necessary details to reduce mistakes and batch variations. There are three main types of documents - commitment documents between industry and regulators, directive documents between management and employees, and record documents between employees and their work. Key documents discussed include master formula records, drug master files, distribution records, and generic drug development requirements. The importance of documentation for regulatory compliance and quality assurance is emphasized.